 Intelligent Design
Intelligent Design
Design for ATP Extends Beyond the Rotary Engine
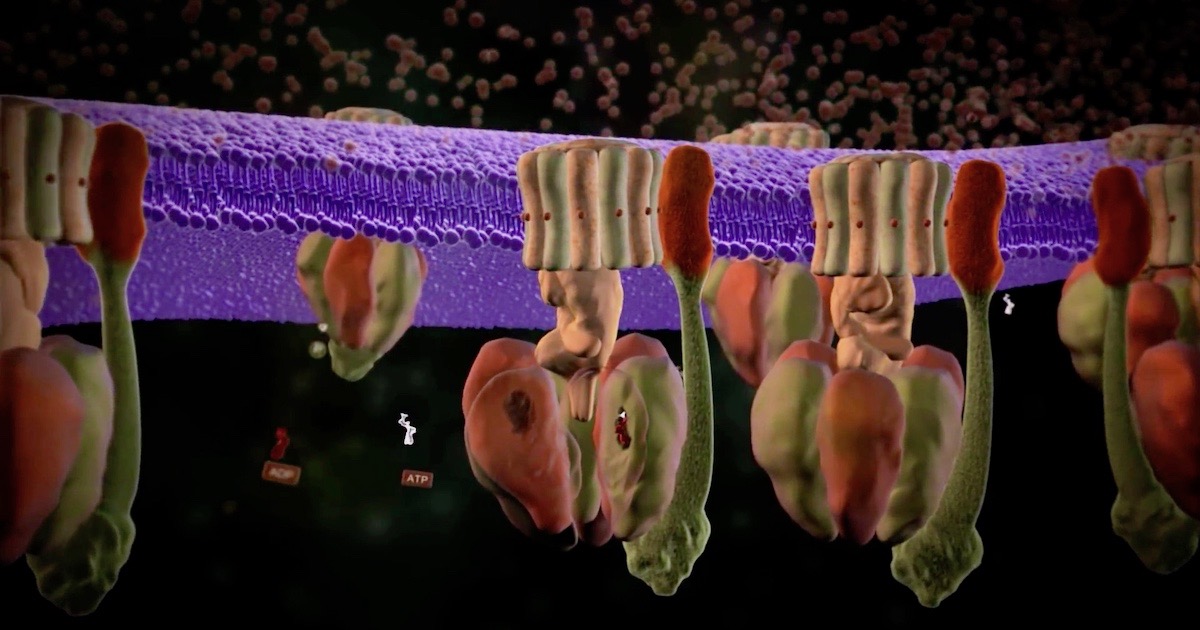
As Michael Behe says in Darwin Devolves, the bacterial flagellum, that icon of irreducible complexity — which has never been explained by a Darwinian process — is one of two rotary engines found in cells. The other is ATP synthase, another icon of intelligent design. Three new papers this month find more things about it to admire. For an introduction, see our animation.
Efficiency Expert
A paper in PNAS by Kwangho Nam and Martin Karplus explores “Insights into the origin of the high energy-conversion efficiency of F1-ATPase.” And do they mean efficiency!
F1-ATPase is a small motor protein, composed of 3 α- and 3 β-subunits that surround a central γ-subunit. The β-subunits alternate cyclically between 2 major conformational states to produce the rotation of the γ-subunit. Although the rotation on the microsecond timescale is powered by the differential binding of ATP and its hydrolysis products ADP and HPO42−, there is near-100% conversion efficiency of the free energy of ATP hydrolysis, which occurs on the picosecond timescale. The free-energy profile constructed for the 360° rotation cycle shows that F1-ATPase achieves its high energy-conversion efficiency by elegantly separating fast catalytic events, which involve small local conformational changes, from the slow binding/release of ligands involved in the large conformational change. [Emphasis added.]
Many learn in physics that 100 percent efficiency is unattainable in energy conversion processes. A theoretically ideal Carnot engine always loses significant energy to heat, and can only hope for perhaps 64 percent efficiency (Penn State). How can ATP synthase achieve near-100% efficiency, such that the energy from one process is completely converted to another, with almost zero loss? Thermal escape is too rapid to overcome, even at this scale.
Almost Zero Loss
The authors found an “elegant separation” between two catalytic events that operate at timescales differing by six orders of magnitude. This apparently gives the motor time for conformational changes in the protein parts and release of products that drive rotation of the rotor. The elasticity or “stiffness” in the rotor also contributes to efficient energy conversion. So finely tuned is each part of the engine to the others, the free energy “changes linearly along the rotation coordinate.” This means that the motor “functions near the maximum possible efficiency.”
In the engines we are familiar with, such as car engines, efficiency rises with temperature. ATP synthase, by contrast, runs with minimal heat loss in both arctic fish living near the freezing point and in thermophilic bacteria living at the boiling point. If human engineers could build car engines with parts that flex a little, instead of using clunky metal pistons and rods, perhaps they would see corresponding increases in efficiency. But it’s doubtful such parts would be able to run as fast or last as long as the proteins in ATP synthase.
How Fast?
A description in BioArchitecture states, “Bacterial enzymes have been clocked to run at up to 42,000 rpm under low load, though for intact enzymes under physiological conditions the number is closer to 6000 rpm.” A typical car starts redlining at that value. High-performance racing cars peak a little above 10,000 rpm. Isn’t it amazing what chance can do?
Molecular machines, like the rotary ATPases described here, seem to have much in common with man-made machines. However, the analogies hold only to a certain point and are in large parts not fully understood. What is evident is that several billion years of evolution have resulted in biological motors that are unsurpassed in efficiency, fine-tuning to their environment and sustainability. Understanding their detailed function at the molecular level is not only important to satisfy our curiosity, but will certainly have implications in understanding human physiology, including mitochondrial disorders, bioenergetics and the processes of aging, as well as impacting nano-engineering and many other fields afar.
Flexible Team
The first paper was concerned primarily with the F1 part of ATP synthase, where ATP synthesis or hydrolysis occurs. The F0 part, where protons drive rotation of a carousel-like wheel, also contributes to the efficiency. It drives the γ-subunit that acts like a camshaft. The camshaft extends into the F1 part, in effect “snapping” ADP and phosphate together to form ATP in three stages per revolution: synthesis, ejection, and loading. A paper by Murphy et al. in Nature explores the flexibility in the γ-subunit that contributes to the efficiency of this “well-oiled machine.”
Biophysicists have long wondered about an apparent mismatch between the F0 and F1 parts of the engine. The F0 carousel is composed usually of 8 to 17 c-subunits, depending on the species, but the F1 synthesis domain has six parts arranged in pairs. Why the non-integer ratio between the domains? Flexibility in the γ-subunit takes care of some of the mismatch by storing elastic energy, but would seem wasteful during continuous rotation. Murphy et al. noticed that the F1 domain actually does take up some of the slack by rotating itself. The opening comment explains:
They solved high-resolution cryo–electron microscopy structures of the ATP synthase complex, extracting 13 rotational substates. This collection of structures revealed that the rotation of the Fo ring and central stalk is coupled with partial rotations of the F1 head. This flexibility may enable the head to better couple continuous rotation with discrete ATP synthesis events.
An animation in the paper shows the F1 domain undergoing a rocking motion back and forth as the F0 domain rotates around continuously. The rocking motion is achieved by means of another finely tuned protein called OSCP. The beauty of this solution allows for F1 heads to accommodate differing sizes of F0 rotors through a universal joint.
We find that the F1 head rotates together with the central stalk and c ring through approximately 30°, or one c subunit, at the beginning of each 120° step. Flexible coupling of the F1 head to the Fo motor is mediated primarily by a hinge at the interdomain link of the oligomycin sensitivity–conferring protein (OSCP) subunit that joins the F1 head to the peripheral stalk. The extended two-helix bundle of the central stalk γ subunit interacts with the catch-loop region of one β subunit of the F1 head. The resulting mechanism of flexible coupling is likely to be conserved in other F1-Fo ATP synthases. Our results provide much-needed context to a wealth of published data indicating that OSCP is a hub of metabolic control in the cell.
The authors conclude:
In ATP synthases, the F1 catalytic head can accompany the rotor through a rotation of ~30° at the beginning of each ~120° step. This movement allows flexible coupling of F1 and Fo. The interdomain hinge of OSCP facilitates flexible coupling and makes this subunit an apposite point for the regulation of ATP synthesis.
Apposite means “suitable; well-adapted; pertinent; relevant; apt.” How apt a choice of word, indeed!
Where the ATP Goes After Synthesis
Some animations of ATP synthesis show the products ejecting from the machine, as if they just fly off into the air. Actually, transport of ADP into and ATP out of the motor are also tightly regulated. The “mitochondrial ADP/ATP carrier” (AAC) is right there, like a UPS truck, to get the products where they are needed.
Inside the mitochondrion, as reported here before, there are inner and outer membranes, with TIM and TOM transporters that control what enters and exits. In another paper in Nature, Bertholet et al. found that AAC transport proteins have more to do than just deliver goods. They actually help regulate how many products get made. In the following, recall that H+ is a proton, the “fuel” in proton motive force that drives F0 rotation in ATP synthase. Protons are supposed to stay in the inner mitochondrial membrane, but being small, they can “leak” out. Can that leakage serve a purpose? Keep your eye on the AAC truck driver:
Here we record AAC currents directly from inner mitochondrial membranes from various mouse tissues and identify two distinct transport modes: ADP/ATP exchange and H+ transport. The AAC-mediated H+ current requires free fatty acids and resembles the H+ leak via the thermogenic uncoupling protein 1 found in brown fat. The ADP/ATP exchange via AAC negatively regulates the H+ leak, but does not completely inhibit it. This suggests that the H+ leak and mitochondrial uncoupling could be dynamically controlled by cellular ATP demand and the rate of ADP/ATP exchange. By mediating two distinct transport modes, ADP/ATP exchange and H+ leak, AAC connects coupled (ATP production) and uncoupled (thermogenesis) energy conversion in mitochondria.
Translating this into a more everyday analogy, the AAC truck driver keeps an eye on how many protons are leaking out into the cytoplasm, and calls back to the engine house to have them slow down production. When the truck driver can keep up with production, proton leakage is small (negative regulation). But when more protons leak out, the driver warns that ATP synthase is outpacing demand.
More Complicated, as Usual
It’s actually more complicated than this, as usual. Proton leakage (IH) is controlled by another protein, Uncoupling Protein #1 (UCP1), that regulates protons crossing the inner mitochondrial membrane. This protein works in partnership with AAC, which is doing its job delivering ATP to the cytosol or bringing ADP back in. The result is another “master”-ful solution to “delicate”-ly regulate the amount of ATP production.
With AAC, IH is negatively regulated by ADP/ATP exchange, whereas with UCP1, IH is simply inhibited by cytosolic adenine nucleotides. This ability to dynamically adjust IH in accordance with ADP/ATP exchange (and thus cellular ATP demand) could make AAC uniquely suited to be the UCP of mitochondria that specialize in ATP production. Thus, AAC appears to serve as a master regulator of mitochondrial energy output, maintaining a delicate balance between ATP production and thermogenesis.
More Details, More Fine-Tuning
As more details of ATP synthase come to light, more and more fine-tuning appears. The synthesis of ATP, necessary from the very start of metabolic life, is now seen to be phenomenally efficient and masterfully regulated by multiple parts working together. Just give chance billions of years, and miracles like this can happen. Not.
Image: A scene from “ATP Synthase: The Power Plant of the Cell,” via Discovery Institute.
