 Intelligent Design
Intelligent Design
The Molecular Flagellar Clutch of Bacillus Subtilis
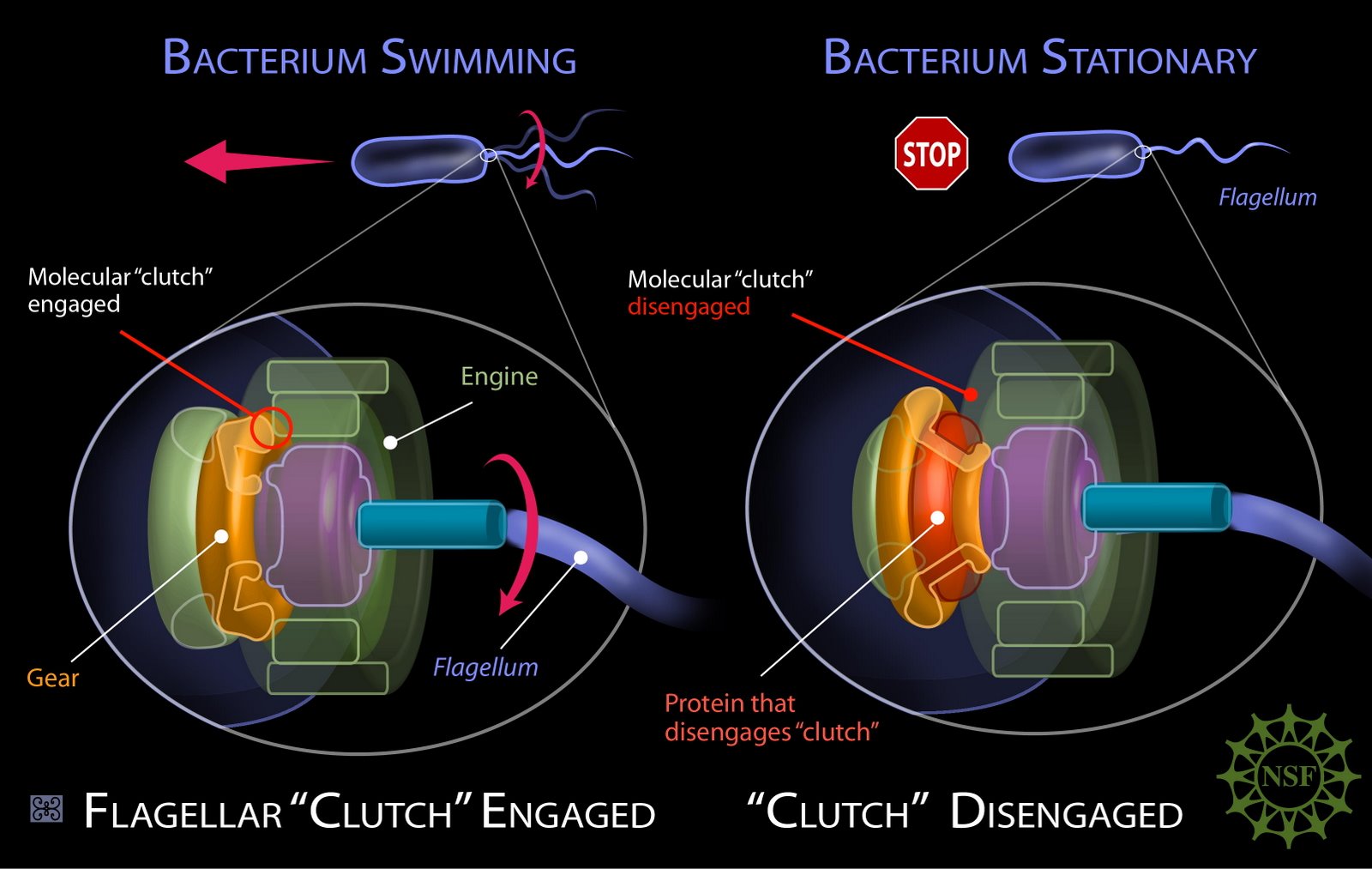
I have previously outlined a few of the key mechanisms undergirding flagellar assembly, and the remarkable process of bacterial chemotaxis by which bacteria change direction in response to chemical signals. There are, of course, a number of different variants in those systems. If you thought that these remarkable mechanisms bear a strong resemblance to an intelligently engineered system, you’re going to love the molecular clutch of Bacillus subtilis (illustrated above), reported by Blair et al. (2008).
Bacillus subtilis is an organism capable of biofilm formation. A biofilm is an antibiotic-resistant aggregate of bacteria, embedded within a matrix of extracellular polymeric substances (EPS) such as proteins and polysaccharides, in which the microorganisms adhere to one another. In a biofilm, bacterial motility is switched off. This raises the question of how this inhibition of motility is achieved. As the authors explain,
The flagellum is an elaborate, durable, energetically expensive, molecular machine and simply turning off de novo flagellum synthesis does not necessarily arrest motility. Once flagellar gene expression is inactivated, multiple rounds of cell division may be required to segregate preexisting flagella to extinction in daughter cells. In contrast, the clutch requires the synthesis of only a single protein to inhibit motility. Furthermore, if biofilm formation is prematurely aborted, flagella once disabled by the clutch might be reactivated, allowing cells to bypass fresh investment in flagellar synthesis. Whereas flagellum expression and assembly are complex and slow, clutch control is simple, rapid, and potentially reversible.
What determines whether the engine of a car is connected to the components that spin the wheels of the vehicle? The answer is the clutch, which ensures that the engine and gears are disengaged. In the flagella of Bacillus subtilis, the analogue to the clutch of a car is a protein called EpsE, which is carried on the same operon (eps) as the genes necessary for EPS formation. EpsE makes contact with the flagellum’s rotor (the component that rotates within the stator). The protein responsible for polymerizing into the rotor is called FliG. The FliG subunits also convert the proton flux energy into the flagellum’s rotational energy. When EpsE interacts with FliG, it triggers a conformational change, causing it to bend such that the rotor is disengaged from the proton-powered engine of the flagellum. Guttenplan et al., (2010) reported that EpsE is bifunctional: it “interacts with the flagella rotor to inhibit motility and also cooperates with other enzymes to synthesize the EPS matrix.”
A related news report stated,
The discovery may give nanotechnologists ideas about how to regulate tiny engines of their own creation. The flagellum is one of nature’s smallest and most powerful motors — ones like those produced by B. subtilis can rotate more than 200 times per second, driven by 1400 piconewton-nms of torque. That’s a lot of horsepower for a machine only a few nms wide.
“We think it’s pretty cool that evolving bacteria and human engineers arrived at a similar solution to the same problem,” said Indiana University Bloomington biologist Daniel Kearns, who led the project. “How do you temporarily stop a motor once it gets going?”
Of course, from an intelligent design standpoint, it is not mysterious at all that bacterial clutch control strongly parallels systems designed by human engineers. I would hesitate to call a system like this “irreducibly complex” (it’s relatively simple and the single enzyme involved is bifunctional). But this need not disincline us from thinking about this system in terms of specified complexity. As blogger David Tyler explains,
The measure of complexity is in the unique shape of the EpsE protein and its ability to engage with the torque-transmitting protein so that power is no longer transmitted.
The system also needs to work in co-operation with a mechanism for disengaging the clutch and reconnecting the motor. The more you learn about the engineering principles involved in biological systems, the more you are compelled to the conclusion that we are indeed dealing with designed systems.
