 Evolution
Evolution
 Intelligent Design
Intelligent Design
Irreducibly Complex: Design of the Type IV Secretion System
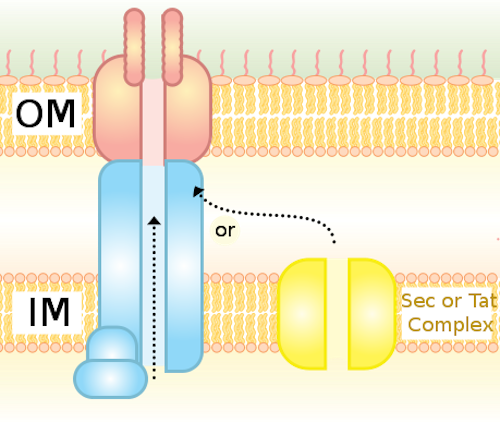
The Type IV Secretion System (T4S) in bacterial cells is a molecular pump embedded in the cell membrane. It’s a very flexible and versatile device, able to pass a wide variety of molecules from cell to cell. These machines “translocate virulence factors into eukaryotic cells, distribute genetic material between bacteria and have shown potential as a tool for the genetic modification of human cells,” according to a paper in Nature. Here are some of the words the researchers use in describing its form and function: complex choreography, intricate, 3 megadalton nanomachine, and remarkable structure. The illustrations in the paper support those adjectives: it’s a beautiful design.
Until recently, not much was known about the T4S because it was difficult to image. Now, with this paper, the structure has come into sharper focus. The authors note a couple of times how different it is from the needle-shaped T3S that was briefly illustrated in the film Unlocking the Mystery of Life.
Our results show a secretion system with markedly different architecture, and consequently mechanism, to other known bacterial secretion systems….
When compared with the type III secretion (T3S) system (the only known structure for an assembled secretion system; Extended Data Fig. 10), the T4S system shows a fundamentally different architecture. While T3S systems are organized as a series of integrated ring-like structures that form a continuous tubular conduit, T4S systems show a more modular design with a two-fold symmetric inner-membrane complex conjoined by a central stalk to a concentric outer membrane channel. Thus T4S systems represent a radically different evolutionary approach, design and mechanism for the translocation of substrate across the bacterial cell envelope.
The T4S machine is composed of 12 different proteins, including 3 ATPases (proteins that spend ATP) that energize it. The parts are arranged, from inner membrane to outer, in the form of two barrels, three tiers, an arch (all these forming the “inner membrane complex” or IMC), a stalk, an I-layer, a middle platform, an O-layer, and a cap (forming the core complex). “Significant flexibility between IMC and the core complex was observed,” they say.
Unlike the T3S, which “has an obvious continuous secretion pore that spans the entire cell envelope,” the “core complex cap” of the T4S “is in an overall closed conformation.” How the substrate gets through is a mystery that will require further work. Usually, Type IV secretions occur between cells in direct contact, so maybe the cap is a protective mechanism like the airlock between two spacecraft in rendezvous. The authors did notice a particularly “remarkable” feature:
Directly above the upper tier of each barrel lies a remarkable structure termed the arch, which interconnects between barrel subunits and the central stalk (Figs 2 and 3d). Each arch comprises a substantial central density that contacts the underlying barrel through up to six thin linkers (Extended Data Fig. 5a).
 Since this was a structural analysis, the authors did not say much about how the machine works. They did mention, though, that the “complex choreography of the substrate through the secretion apparatus” includes a two-step process. The substrate would first cross the inner membrane with the help of coupling proteins, then would be channeled into the core complex and extruded. “Evidence is also mounting for interaction” with three other proteins. They even invoked the analogy of gears: “VirB4/TrwK hexamers could recruit, or even be replaced by, VirB11/TrwD or VirD4/TrwB, which would allow different enzymatic and functional gearing of the T4S machinery during the secretion cycle.” This seems to suggest that the pump can adapt its internal structure to the type of substrate passing through.
Since this was a structural analysis, the authors did not say much about how the machine works. They did mention, though, that the “complex choreography of the substrate through the secretion apparatus” includes a two-step process. The substrate would first cross the inner membrane with the help of coupling proteins, then would be channeled into the core complex and extruded. “Evidence is also mounting for interaction” with three other proteins. They even invoked the analogy of gears: “VirB4/TrwK hexamers could recruit, or even be replaced by, VirB11/TrwD or VirD4/TrwB, which would allow different enzymatic and functional gearing of the T4S machinery during the secretion cycle.” This seems to suggest that the pump can adapt its internal structure to the type of substrate passing through.
In summary, the T4S, with its 12 protein parts, represents another irreducibly complex, ATP-powered mechanism for pumping a wide range of molecules from inside to outside the cell. Throughout the paper, the authors employ the language of machinery. The parts work together like machines to perform a function.
Some Darwinian evolutionists (e.g., a 2004 paper in Nature Reviews Microbiology) try to make the T4S homologous with the “conjugation machinery” in bacteria. This is another example of the “co-option” argument discussed in Unlocking the Mystery of Life. The argument tries to account for one machine by saying it borrowed parts from another machine. But as Scott Minnich responded in the film, where did those parts come from? You can only run that argument so far, he says, until you wind up borrowing parts from nothing. Co-option also fails to account for the assembly instructions, which are even more complex than the parts. No one has produced a detailed path by which the T4S could be reached via random mutations. Unguided evolution fails, therefore, to explain the origin of the T4S.
Is the T4S an “evil” design since it is involved in transferring pathogens? Virulent bacteria use these systems to spread terrible human diseases like whooping cough, Legionnaire’s disease and stomach cancer. Some bacteria spread plant diseases with these machines. But other bacteria use them for everyday functions, like conjugation, information sharing or taking up DNA from the environment. Are the pathogenic forms examples of good machines gone bad? Intelligent design theory is only concerned with judging whether something is designed or not. It leaves moral questions in the capable hands of theologians and philosophers.
If Darwinists were already struggling to connect the T3S to the bacterial flagellum in some evolutionary scenario, they are now faced with a “radically different evolutionary approach, design and mechanism” to explain by unguided natural processes. We prefer to say that machine language is design language.
