 Evolution
Evolution
 Intelligent Design
Intelligent Design
Enzymes Are Essential for Life; Did They Evolve?
Editor’s note: We are delighted to welcome a new contributor, the esteemed microbiologist Olen R. Brown. Among other distinctions, Dr. Brown is Professor Emeritus at the University of Missouri.
Darwinian evolution, even in its 21st-century form, fails the formidable task of explaining how the first enzyme arose. Evolution also fails to explain how the first enzyme was changed into the approximately 75,000 different enzymes estimated to exist in the human body or the 10 million enzymes that are thought to exist in all of Earth’s biota. Join me in a legitimate process in science, one made popular by Albert Einstein. It’s called a Gedanken — a thought experiment. Let us see if evolution meets the challenge of logic required if it is to explain how enzymes came to be.
Enzymes have what seem to be near-miraculous abilities. They are catalysts that greatly accelerate reactions by providing an alternate reaction pathway with a much lower energy barrier. Thus, although they do not create new reactions, they greatly enhance the rate at which a particular substrate is changed into a particular product. Every chemical reaction in the cell that is essential to life is made possible by an enzyme. Richard Wolfenden has concluded that a particular enzyme required to make RNA and DNA enormously speeds up the process.1 Without the enzyme, this reaction is so slow that it would take 78 million years before it happened by chance. Another enzyme, essential for making hemoglobin found in blood and the chlorophyll of green leaves, enormously accelerates an essential step required for this biosynthesis. Wolfenden explains that enzyme catalysis allows this step in biosynthesis to require only milliseconds but 2.3 billion years would be required without the enzyme. These enormous differences in rates are like comparing the diameter of a bacterial cell to the distance from the Earth to the Sun.
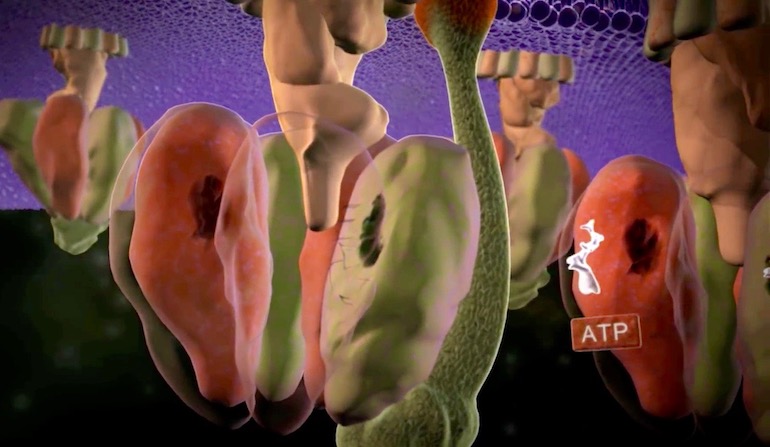
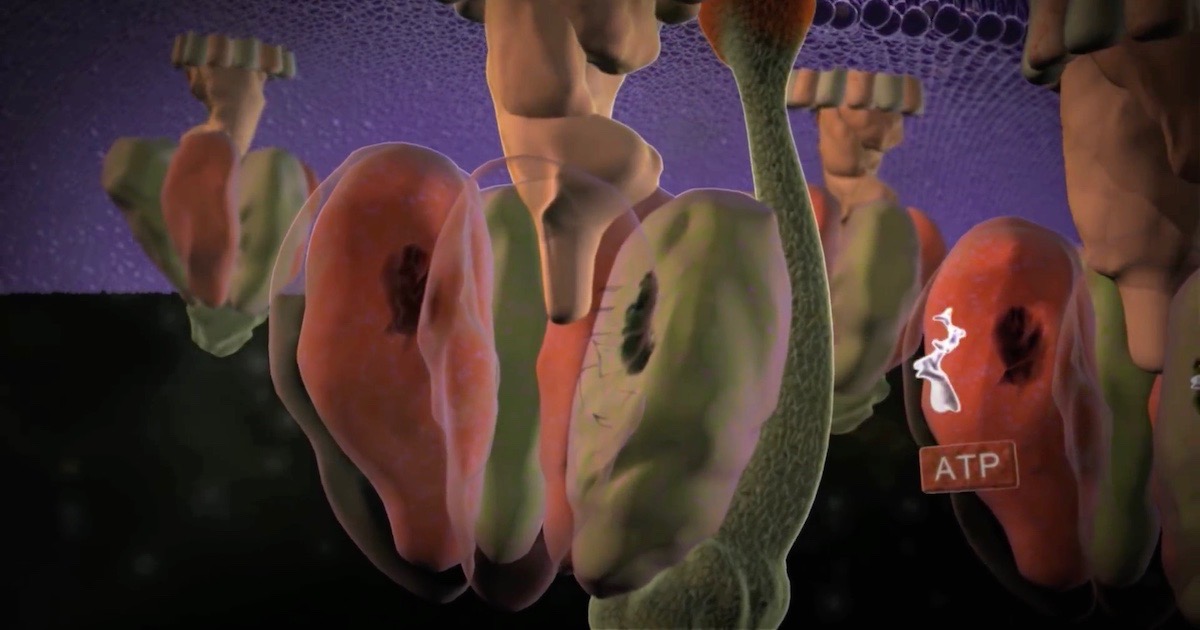
Regenerating ATP
Think of the enormous number of different chemical reactions required for life. Now, focus on only one such reaction, the need to regenerate ATP — the energy source for all life processes. As Lawrence Krauss has written, “The average male human uses almost 420 pounds of ATP each day … to power his activities… there is less than about 50 grams of ATP in our bodies at any one time; that involves a lot of recycling… each molecule of ATP must be regenerated at least 4,000 time each day.”2 This means that 7 x 1018 molecules of ATP are generated per second. By way of comparison, there are estimated to be only 100 billion stars (1 x 1011) in our galaxy, the Milky Way. To efficiently regenerate a molecule of ATP (recharging the cell’s battery) requires a specific enzyme. To create a molecule of ATP is even more complex. The citric acid cycle is only one important part of this process and it has eight enzymes. As the name “cycle” implies, these enzymes must function in sequence. The absence of any one enzyme stops the process. How the interdependent steps of a cycle can originate is left without explanation. Life in Darwin’s hopeful, warm, little pond is dead in the water.
Obviously, in seeking to explain how enzymes arose and diversified, the evolutionist must use the processes of evolution. It is proposed, and I concede it as possible, even logical, that the gene coding for an essential enzyme could be duplicated and the duplicated gene could be expressed as a mutation. The protein coded by this duplicate gene might catalyze a slightly different reaction. There are known examples, but they produce only slight differences in products or reaction mechanisms. Consequently, a mutation can introduce only very limited changes in a specific protein. This limits the scope of change to triviality compared to the scope required by evolution.
“There Was a Little Girl…”
I am reminded of a poem. “There was a little girl, and she had a little curl, right in the middle of her forehead. When she was good, she was very, very good, and when she was bad she was horrid.”3 The power of genetic mutation as a source of change for evolution is like the little girl in this poem. It is good (even very good) at explaining what it can explain — trivial changes — but horrid at explaining any changes needed for the evolution of species. Likewise, natural selection is ineffectual as an editor for evolution. (See “Gratification Deferred”,4 in my book The Art and Science of Poisons.)
Thus, scientific evidence is entirely lacking for the notion that enzymes arose by chance. The idea is ludicrous. This is true even if a primeval solution contained all the twenty amino acids of proteins but no genes and no protein-synthesizing machines. Ah, but, you may say, the Nobel laureate George Wald has written5: “Most modern biologists, having reviewed with satisfaction the downfall of the spontaneous generation hypothesis, yet unwilling to accept the alternative belief in special creation, are left with nothing.” He also wrote (on the same page): “One has only to contemplate the magnitude of this task to concede that the spontaneous generation of a living organism is impossible. Yet here we are as a result, I believe, of spontaneous generation.” In the same article he wrote: “Time is in fact the hero of the plot… the ‘impossible’ becomes possible, the possible probable, and the probable virtually certain. One has only to wait: time performs the miracles.” To be fair, Wald puts the word “impossible” in quotation marks. One may believe this, but surely it is beyond the logical meanings of words and concepts — and Wald appeals to “miracles,” does he not?
Time for a Gedanken
With this quandary, should not today’s scientist be allowed a Gedanken? Formerly, from at least the time of Aristotle, it was generally thought that organic substances could be made only by living things — one definition of vitalism. Frederick Wöhler’s laboratory synthesis of urea in 1828 was startling evidence incompatible with this idea. Perhaps a new look at the essential differences between life and non-life could be instructive. Not to be misunderstood, I do not mean to advocate for vitalism in its old sense, but simply to recognize the vast differences between non-living matter and a living cell in light of today’s knowledge of molecular biology. That a void exists in understanding this difference cannot be denied.
Astronomy, physics, and chemistry have laws that are useful for calculating and making predictions and, to a degree, even as explanations. The high school student can state the law of gravity and make calculations. The professor can do little more to explain this, or any, law of nature. Are not all natural laws, considered as explanations, only tautologies in the end? How is information content that is designed in a law made manifest in nature? Do laws disclose innate properties of energy, matter, and space-time?
Scientists are permitted to propose laws that mathematically describe fundamental particles and their behaviors without being stigmatized as appealing to the supernatural. Why should this not be acceptable in biology? Let us ponder the origin of enzymes and begin a conversation about the laws required to permit complexification in life. A starting place is the admission that perhaps things that appear to be designed are, in fact, designed. How this design occurs is surely a subject for scientific investigation, no more to be disallowed than is the use of laws in physics, astronomy, and chemistry to guide mathematical understanding of complex outcomes.
Notes:
- Richard Wolfenden. “Without enzymes, biological reaction essential to life takes 2.3 billion years.”
- Lawrence M. Krauss. Atom, an Odyssey from the Big Bang to Life on Earth… and Beyond. 1st ed., Little, Brown and Company 2001.
- Henry Wadsworth Longfellow. “There Was a Little Girl,” Vol. I: Of Home: Of Friendship, 1904.
- Olen R. Brown. The Art and Science of Poisons. Chapter 3, “The Dawnsinger.” Bentham Science Publishers.
- George Wald. “The Origin of Life,” Scientific American, August 1954, pp 46, 48.
Image source: “ATP Synthase: The power plant of the cell,” via Discovery Institute.
