 Intelligent Design
Intelligent Design
Membrane Channels Show Astonishing Specificity
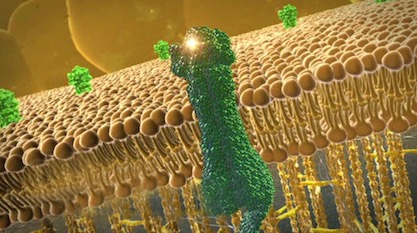
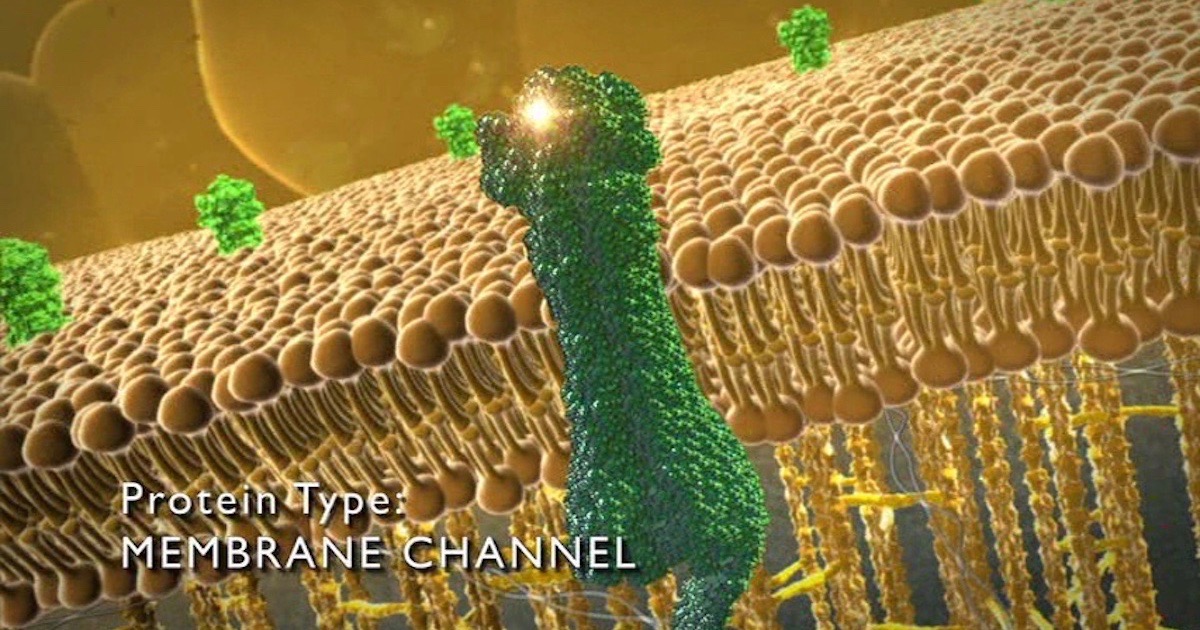
One key difference between life and non-life is active transport. Differing from natural osmosis or diffusion, which tends to move molecules from areas of high concentration to areas of low concentration, active transport is achieved through molecular machines able to recognize and selectively permit passage, even against a strong concentration gradient. Like Maxwell’s demon, membrane channels seem to violate the laws of physics. No laws are violated, of course, any more than turnstiles at Disney World violate physics to keep people out who didn’t buy a ticket. In both cases it’s the programming and placement of specific parts that allows mechanisms to go against what would otherwise occur by unguided nature.
The improving quality of imaging techniques is allowing scientists to peer deeper into the mechanisms of membrane channels. Different channels work in different ways. All have some kind of “selectivity filter” to allow passage of desired molecules and exclude others. Here are a few new discoveries.
The Big Potassium Channel
Biophysicists at the University of Massachusetts at Amherst have cracked the selectivity filter of one type of ion channel, the “big potassium” (BK) channel. This channel operates very rapidly in muscle and nerve cells. Chains of these channels in the cell membrane “fire” in rapid succession, creating a wave of information that can pass from stubbed toe to brain in a fraction of a second. Scientists had expected that this channel would operate some kind of gate that opens and closes, as in other channel types. But before now, they were baffled about why the large central pore of the channel appeared to remain open, even when potassium atoms were not entering.
This puzzle led the UMass team to discover an entirely new mechanism they call “hydrophobic dewetting,” which is “drastically different from what has been observed in other ion channels.” A change in calcium binding in the channel makes water molecules bead up as they do on an oily surface. “When the BK pore is oily, the water forms a vapor phase that acts like a barrier and prevents all ions from entering,” says Jianhan Chen, a member of the team that solved the puzzle, using computational modeling tested against real-world mutagenesis studies.
He says, “If you think about why nature might want to use a vapor barrier where there is a big pore that has to carry a lot of electrical current, to apply a physical barrier you would need a protein structural re-arrangement which would probably be either too big or too slow, or both. In a way, nature is really clever in using this hydrophobic dewetting phenomenon to create a sensitive and fast gate. We were actually really surprised to see that the changes in pore shape and surface properties are relatively small and subtle, but they have big consequences on its hydration properties.” [Emphasis added.]
The open-access research paper, in Nature Communications, expresses surprise at this unique yet very effective mechanism:
The gating mechanism of transmembrane ion channels is crucial for understanding how these proteins control ion flow across membranes in various physiological processes. Big potassium (BK) channels are particularly interesting with large single-channel conductance and dual regulation by membrane voltage and intracellular Ca2+. Recent atomistic structures of BK channels failed to identify structural features that could physically block the ion flow in the closed state. Here, we show that gating of BK channels does not seem to require a physical gate. Instead, changes in the pore shape and surface hydrophobicity in the Ca2+-free state allow the channel to readily undergo hydrophobic dewetting transitions, giving rise to a large free energy barrier for K+ permeation.
The Voltage-Gated Proton Channel
Another ion channel named HV1, vital to health of everything from sea squirts to humans, is able to discriminate protons. “The gating of HV1 is evidently more complicated than we imagined,” concludes Thomas E. DeCoursey in a research review in the Proceedings of the National Academy of Sciences (PNAS). He discusses recent findings from two studies on HV1 that appear to indicate that coordinated conformational changes take place between subunits in the selectivity filter before a proton can pass. This “cooperative gating” suggests that “each channel must undergo multiple voltage-dependent transitions between closed states before it opens.” It should not seem surprising that a channel able to control passage of atomically-small hydrogen ions must operate with the utmost precision and care. Incidentally, DeCoursey notes that a single eosinophil cell in the blood stream has as many as 40,000 of these HV1 channels.
The Mitochondrial Uniporter
This channel, found in mitochondrial membranes, must discriminate between calcium (Ca) and manganese (Mn), which are only 5 atomic numbers apart in the periodic table. Failure of the uniporter to discriminate between their doubly-charged ions (Ca2+ and Mn2+) can lead to “manganese toxicity” that is implicated in neurodegenerative disabilities like Parkinson’s disease.
Ca2+ activates the “uniporter machinery,” says a team of seven primarily from Howard Hughes Medical Institute and Harvard, reporting in PNAS. Notice the irreducible complexity required to give this important transport channel its exquisite selectivity and ability to resist a steep concentration gradient of unwanted ions:
The mitochondrial uniporter is a Ca2+-activated Ca2+ channel in the organelle’s inner membrane. It exhibits both remarkably high conductance and high ion selectivity. It is capable of discriminating between Ca2+ and other cations, even those that are three to six orders of magnitude more abundant, such as K+ or Mg2+. Typically, ion selectivity of the conductance in Ca2+ channels is afforded by the selectivity filter, a narrow passage in the channel’s pore that contains acidic amino acids poised for chelating the conducted ion. This form of uniporter’s selectivity is conferred by a highly conserved “DXXE” motif located at the intermembrane space-facing apex of the pore. However, for the mitochondrial uniporter, calcium is not only the transported ion, but it also acts as the regulatory ligand by engaging the EF hands of the uniporter’s regulatory subunit MICU1. In principle, and as we demonstrate in this paper, the ion selectivity at the ligand-gating site can significantly contribute to the overall ion selectivity of the channel complex.
This channel must allow rapid conductance of calcium, but resist manganese. How does it do it? “We conclude that the remarkable ability of the uniporter to discriminate between Ca2+ and Mn2+ resides, in part, in the regulatory MICU1–MICU2 complex.”
Experiments on the lab roundworm C. elegans showed that the MICU1 regulator can bind to either ion, but only the calcium ion triggers a conformational change. It acts as a checkpoint, therefore, to ensure that manganese does not pass:
In the current study, we have demonstrated that MICU1 provides a checkpoint that enables the mitochondrial uniporter complex to discriminate between Ca2+ and Mn2+. When MICU1 is absent, both Mn2+ and Ca2+ can pass freely through the uniporter because the conductance is not highly selective for Ca2+ over Mn2+ and the uniporter’s gating selectivity is lost. In the holo-complex, Mn2+ transport is blocked unless Ca2+ engages the EF hands of MICU1 to induce a specific conformational transition.
Conclusion
This was just a brief look into three different mechanisms in three different channels that facilitate active transport. There are dozens of channel families, collectively offering hundreds of different molecular machines vital for life.
A cell that cannot control what comes in or leaves its interior cannot survive. Science should reject all unrealistic hypotheses that picture simple bags of molecules trying to become cells. Oparin tried that in the 1920s, and some are trying to keep his dream alive (Astrobiology Magazine). If the simplest conceivable cell requires channels as complex as these, what is to be expected for the other requirements, such as a genetic code, metabolism, and a membrane? Clearly, such complex mechanisms, all working together in harmony, are far, far beyond the reach of chance. Coordinated, hierarchically-arranged “functional wholes,” as Douglas Axe calls them in his book Undeniable, are hallmarks of intelligent design.
Image credit: Illustra Media, from Origin: Design, Chance and the First Life on Earth.
