 Intelligent Design
Intelligent Design
Dazzling Acts in the Cell Circus
Intelligent design is evident at all scales, from the whole universe down to the single atom. This observation puts evolutionists on the defense: how are they going to explain this appearance with their limited resources of mindless chance and natural law? They can’t. We live in a meaningful world of purpose, design, and beauty that can only be explained by intelligent agency. There is perhaps no better place to illustrate this than the living cell. Here are some of the latest findings about cellular machines — the greatest design show on earth at the nanometer scale.
Flagellum Optimization
Beginning with our old design standard, we find that science continues to unveil more engineering elegance in the bacterial flagellum. In PLOS Biology, fourteen scientists show that “Hook length of the bacterial flagellum is optimized for maximal stability of the flagellar bundle.” The hook — that bend in the shaft just outside the cell membrane from which the filament extends — cannot function properly if it is not the right size. Isn’t it amazing how blind chance pulled off this optimizing trick right at the function junction?
We conclude that too-short hooks may be too stiff to function as a junction and too-long hooks may buckle and create instability in the flagellar bundle. Accordingly, peritrichously flagellated bacteria move most efficiently as the distance travelled per body rotation is maximal and body wobbling is minimized. Thus, our results suggest that the molecular ruler mechanism evolved to control flagellar hook growth to the optimal length consistent with efficient bundle formation. The hook-length control mechanism is therefore a prime example of how bacteria evolved elegant but robust mechanisms to maximize their fitness under specific environmental constraints. [Emphasis added.]
Locator Beacons
The T cells of the human immune system are constantly on the prowl for invaders. When they find these “antigens” they need a way to tag them for destruction. Scientists publishing in Nature Communications found that tiny protrusions on the T cells, called microvilli, aren’t just there for decoration. They “constitute immunological synaptosomes that carry messages to antigen-presenting cells.” Moreover, the messaging is bidirectional.
In this study, we observe that single T cell contacts with APCs [antigen-presenting cells] occur through microvillar extensions, which appear to serve as locations for sequestration of immunologically important molecules, including TCR complexes [T cell receptors], costimulatory and adhesion molecules, and various cytokines. We find that microvilli are separated from the T cell body by the combined action of two independent mechanisms (trogocytosis [i.e., gnawing or nibbling] and membrane budding) and are deposited at the surface of cognate APCs, thereby potentially acting as an effective means of delivering T cell messages to cognate APCs. Consistent with this potential role, these T cell microvilli-derived particles (TMP) are independently capable of activating cognate dendritic cells (DC). Therefore, our findings suggest that T cell microvilli might serve as “immunological synaptosomes” [i.e., communication transport bodies] with TMPs as a class of membrane vesicles serving as conveyors of T cell messages or traits to cognate APCs.
The biological role of microvilli “has remained surprisingly unrecognized,” the authors note. They are not simple spaghetti-like protrusions. They “contain many parallel bundles of actin filaments that extend the cell membrane in the form of a finger.” TCRs cluster at the tips, making them “effective sensors for antigenic moieties on APCs or target cells.” Dendritic cells “are responsible for the initiation of adaptive immune responses and hence function as the ‘sentinels’ of the immune system” (British Society for Immunology).
We’re seeing the details of a whole military system equipped with sophisticated machinery to protect the body. Consider a Star Wars analogy. Imagine a roaming defense craft encountering an enemy spaceship. Not being sufficient on its own to destroy it, it dispatches a device that attaches to the hull of the ship. The device is equipped with machinery to identify the craft and certify its intentions. Then, it leaves a beacon that the star fleet can identify: ‘This is an enemy craft; call in the fighters.” Isn’t it marvelous how evolution figured out this all out?
In conclusion, lymphocytes are highly specialized cells that circulate throughout the entire body to scan a specific antigen and deliver messages to target cells. Because of their dynamic motility, lymphocytes might have evolved to harbor specialized mechanisms to promote communication between interacting cells, even under mobile conditions. Therefore, microvillus-derived message transfer might be a unique means of communication for lymphocytes or immune cells and not cells occupying tissues.
Wind-Driven Monorail
Could you operate a monorail without energy? Cells do it. Most activity in the cell requires the energy molecule ATP, but scientists at the Tokyo Institute of Technology have found an enzyme that moves directionally like a monorail train without a battery or engine. This news item describes “Chitinase as ‘burnt-bridge’ Brownian monorail efficiently hydrolyzing recalcitrant biomass.” The technique is helpful to the enzyme, which operates in the extra-cellular spaces where ATP is less available. The enzyme breaks down crystalline chitin, one of the major contributors to biomass on the earth after cellulose. It’s a tough molecule to degrade. Chitinase proceeds stepwise through the crystal and turns it into a water-soluble product.
At the microscopic scale of cells, Brownian motion jostles things about like random breezes. A monorail train on a track could take advantage of free wind energy, even if random, by moving when the wind blows it the way it wants, then burning the track behind it. “This is so called ‘burnt-bridge’ mechanism, removing the rail for backward movement and forcing a molecule to move forward.” Look at the illustration where an artist shows chitinase on its track next to a modern monorail train. It’s an inspiring trick (thanks to evolution):
The finding demonstrates how SmChiA controls the Brownian motion and extracts fast unidirectional motion for continuous degradation of crystalline chitin without dissociation. The strategy evolved by SmChiA can be applied not only to engineer chitinases and cellulases for more efficient chitin and cellulose degradations, but also to design fast-moving artificial molecular motors such as DNA walkers.
Safe Industry
Study the history of industry, and you learn about terrible accidents: explosions, toxic substance releases, and catastrophic effects on the environment. Industries that regularly handle toxic substances are especially prone to disasters. Only by intelligent analysis of the causes of accidents, and the introduction of safe practices, have industries and governments been able to minimize the risk. Your body, though, already knows about industrial safety. An announcement from the American Society for Biochemistry and Molecular Biology answers “How cells handle a sticky, toxic, but absolutely essential molecule.”
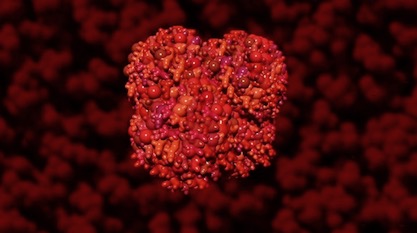
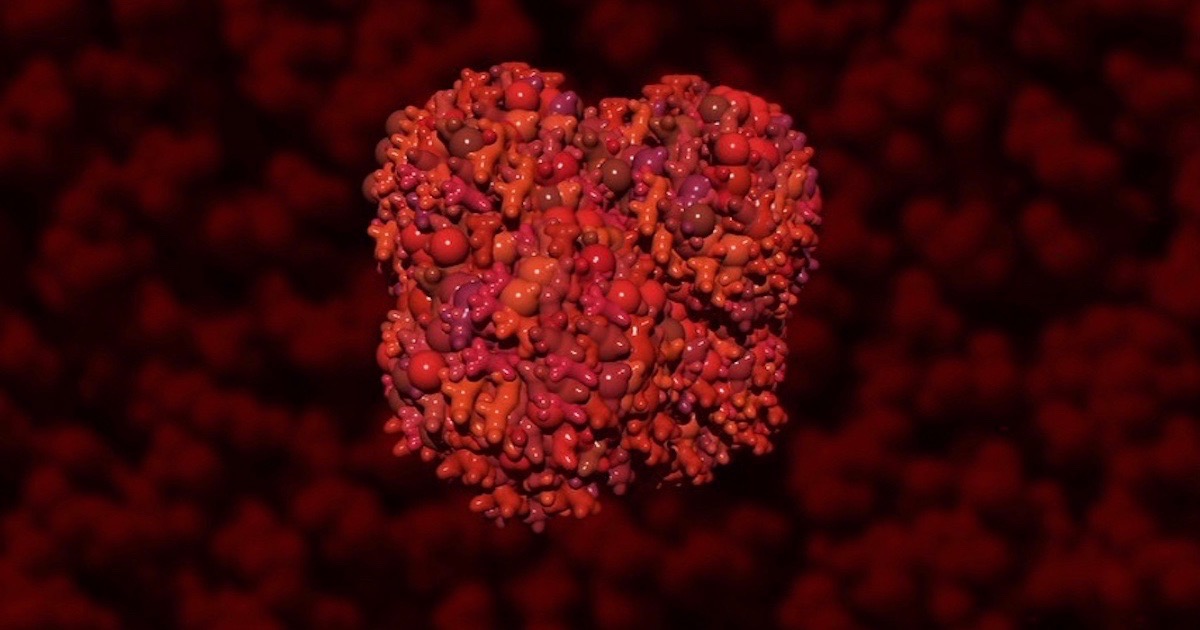
That molecule is heme. Remember hemoglobin, the oxygen-carrying molecule in blood? “On its own, heme is toxic and reactive, but when slotted correctly into certain proteins, it’s absolutely essential,” they say. Heme is also sticky, easily binding to the wrong things. It must be handled with great care. If you like to breathe air, be thankful for safe practices in your cells for handling heme. Be especially thankful for one boring, ‘unglamorous’ chaperone protein tasked with ferrying this toxic molecule to the right places.
GAPDH is an enzyme involved in breaking down sugar in cells. It’s a commonplace, unglamorous component of the cell’s basic metabolism, so much so that laboratory scientists mainly use it as a basic control in studies of other proteins.
“GAPDH is such a ridiculous candidate,” [Dennis] Stuehr said. “But there’s been this emerging story that GAPDH isn’t just this boring glycolytic enzyme that’s in every cell; it has these other roles in cell biology. And heme delivery is one of these new roles.”
Manufactured in mitochondria, heme must be delivered to downstream targets. GAPDH “not only binds heme, and binds a lot of it, but is also required for delivery to downstream heme protein targets.” It’s an unglamorous job, but someone has to do it. And since GAPDH is well equipped for this dirty job, we can all breathe easier.
Image: Hemoglobin, Illustra Media, from Origin: Design, Chance and the First Life on Earth.
