 Intelligent Design
Intelligent Design
Orderly Disorder: A Molecular Motor Regulated by IDP
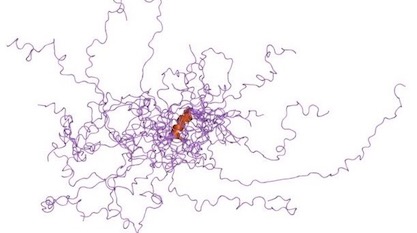
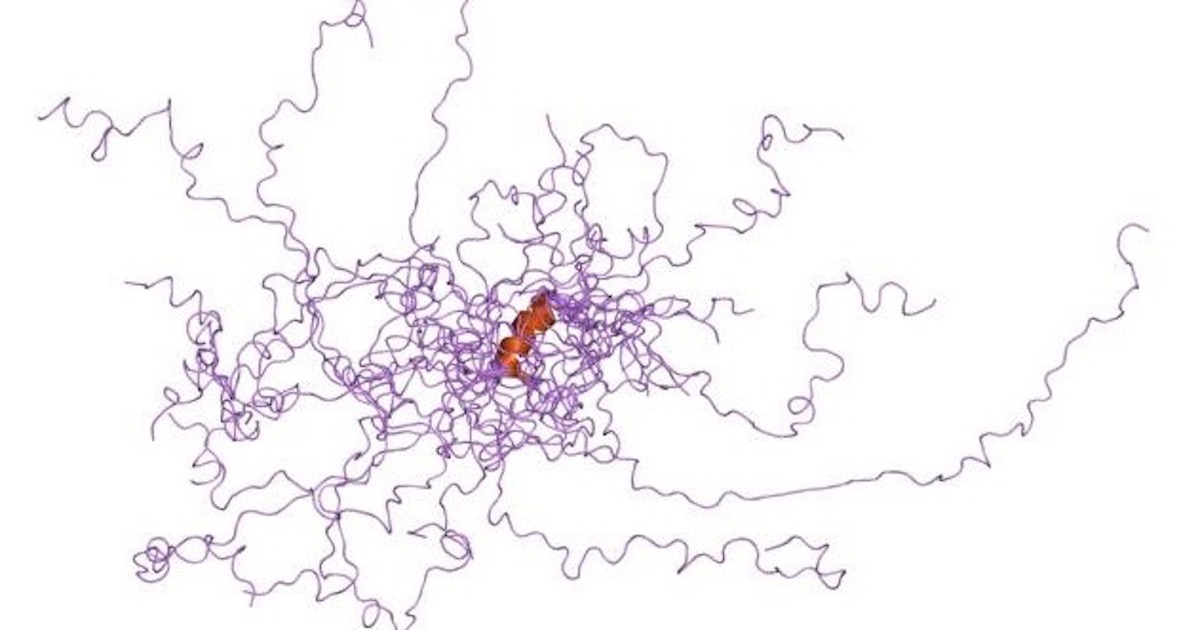
Proponents of intelligent design see significance in the observation that molecular machines require a precise sequence of amino acid residues to fold into functional shapes. The “sequence hypothesis” was a primary argument in Douglas Axe’s book Undeniable and in Stephen Meyer’s Signature in the Cell, where both authors focus on the high degree of specificity required in proteins. Only a tiny fraction of amino acid sequences in the universe of sequence space will produce foldable proteins — and proteins need to fold into three-dimensional architectures in order to function.
What, though, are we to make of the fact that cells contain large numbers of “intrinsically disordered proteins” (IDPs) that flop around in the cytoplasm without folding? Boreiakaite et al. write in PNAS, “IDPs are characterized by a lack of defined structure and they populate many different conformational states.” These polypeptides do not fold. Are they junk? Are they mistakes? Are they evolving? Are they transitional forms? What are they there for? Prepare for a major turnaround in thinking. Here’s a case where an IDP turns into a functional machine on the fly, regulating a rotary engine like an emergency brake. In fact, the disordered part makes it “exquisitely sensitive” to environmental conditions, only switching into its functional role when needed. It’s like an automatic brake that kicks in when it senses the car slipping downhill.
A Quiet Revolution
Five years ago, Jonathan Wells considered IDPs a case to watch. From “Biology’s Quiet Revolution” here at Evolution News:
In 1996, biologists discovered a protein that does not fold into a unique shape but can assume different shapes when it interacts with other molecules. Since then, many such proteins have been found; they are called “intrinsically disordered proteins,” or IDPs. IDPs are surprisingly common, and their disordered regions play important functional roles. [Emphasis added.]
Wells did not give any detailed examples at the time, but mentioned two scientists who were looking at “assemblages” of IDPs to figure out what they do. He seconded their efforts, because “huge unanswered questions remain” about proteins in cells. The one we will look at is pretty spectacular. It may lead to paradigm shifts about the relationship of sequence and function.
Before we look at this case in detail, recall our good friend ATP synthase. It’s a tiny irreducibly complex rotary engine that keeps us alive. This rapidly spinning motor, powered by protons as our animation shows, pumps out ATP energy “coins” for almost everything cells need to do. There’s one problem; it is reversible. It can just as easily spin backwards, spending valuable ATP to output protons. It’s like the car that can roll downhill as easily as it rolls uphill. In some cases, that is good, but something needs to regulate the direction needed. Here comes an IDP to the rescue:
Adenosine triphosphate (ATP), the fuel of biology, is produced by a molecular machine with a rotary action. Inside the mitochondria of eukaryotic cells, rotation is driven by a proton-motive force across the inner membranes of the organelle generated by oxidation of sugars and fats in food. Under anoxic conditions, the rotary machine hydrolyzes ATP and reverses rotation. This wastage is prevented by an intrinsically disordered region of the inhibitor protein, IF1, which inserts itself in the machine and stops reverse rotation. The inhibitory activity of IF1 is regulated by self-association, which is influenced by pH and ion-types, providing a potential molecular mechanism for the modulation of ATPase activity by cellular physiology via this solution-responsive, self-associating protein.
To the Rescue
In simple language, this disordered protein IF1 acts like an emergency brake when the pH changes, which would otherwise waste precious ATP and turn the machine into a proton pump. But how does it do that?
If the pmf [proton motive force] is dissipated, for example, during ischemia, the enzyme reverses its direction of rotation and starts to hydrolyze ATP, but this hydrolytic activity becomes inhibited by a protein known as IF1.The active state, which is present at pH values around neutrality, binds to one of the three catalytic sites of the membrane extrinsic F1-domain of the enzyme, stopping hydrolysis.
This binding action, they explain, is pH dependent. At a certain point when acidity increases, disorder changes to order:
In solution, the dimerization of dimers and occlusion of the inhibitory region is pH-dependent. At pH values below about 7.5, IF1 is an active dimer, and at higher pH values, tetramers and oligomers form, and IF1 is inactive. The mutation H49K (10) abolishes the ability to form tetramers, and the mutated dimeric IF1 is constitutively active and pH-independent…. In solution, the C-terminal region of IF1 forms a dimeric α-helical coiled-coil on its own, whereas the N-terminal inhibitory region is unstructured and provides an example of an intrinsically disordered protein (IDP). During inhibition of F1-ATPase, the disordered region interacts with the most open of the three catalytic sites and becomes α-helical from residues 31–49. Hydrolysis of two ATP molecules converts this open site with bound IF1 to, first, a partially closed site, where residues 23–50 of IF1 are α-helical region, and then to the fully closed state, where the inhibitory region is structured to its greatest extent.
IF1 is, therefore, disordered when it needs to be inactive (in neutral and alkaline conditions), and converts to an ordered form when the pH becomes more acidic, which would cause the motor to go into reverse (i.e., to hydrolize ATP). Thus, “wastage” is prevented by this shape-shifting IDP that becomes ordered only when environmental conditions require it to function. Just how finely tuned is this “disordered” protein for its role?
We identified the equilibrium between dimers and tetramers as a potential central factor in the in vivo modulation of the inhibitory activity and suggest that the intrinsically disordered region makes its inhibitory potency exquisitely sensitive and responsive to physiological changes that influence the capability of mitochondria to make ATP.
They use that word “exquisite” again later on: “the intrinsically disordered nature of IF1 makes its inhibitory potency exquisitely sensitive and responsive to any physiological changes that may influence the capability of the mitochondria to make ATP.” This IDP is disordered on purpose!
One of the co-authors of this paper is John E. Walker, who shared the Nobel Prize in Chemistry in 1997 for figuring out the rotary action of ATP synthase. Here he is, 22 years later, still fascinated with this motor. The inhibitory role of IF1 has been known for years, but this new paper shows how it works: changes in environmental pH make the IDP switch into an ordered state that binds to the motor and stops it, preventing wastage of ATP.
Implications for Intelligent Design
ATP production by ATP synthase goes on continuously, even in our sleep. It’s been said that an active person generates his or her body weight in ATP each day. If production stopped, a person would be dead before hitting the floor. ATP synthase, a prime example of irreducible complexity, is present in all living cells — even the earliest “primitive” cells. The rules of probability render it vanishingly unlikely that chance could produce such a wonderfully efficient molecular motor.
The earliest cells would have needed more than just the motors, though. They would have needed regulators to sense and control the direction of rotation of the machines, too, or else they would have died when conditions changed. Scientists are now beginning to learn that intrinsically disordered proteins are not really disordered at all, but work as environmental sensors and regulators that are “exquisitely sensitive and responsive” to conditions. Will other IDPs be found to quickly change from flopping strands into functional regulators based on environmental changes? Will the DNA sequences that produce IDPs continue to confirm the sequence hypothesis?
Design-theoretic biology is poised to find out. If Darwinism had been helpful in understanding the function of IF1, the authors surely would have mentioned it. But they don’t. The “revolution in biology” that Wells saw coming is advancing by scientists who seek to understand the purpose for things, even when the purpose is not apparent. Understanding accelerates when research begins with Paul Nelson’s dictum: “If something works, it’s not happening by accident.”
Image credit: Jawahar Swaminathan and MSD staff at the European Bioinformatics Institute [Public domain], via Wikimedia Commons.
