 Intelligent Design
Intelligent Design
Cell Cannibalism Shows Intelligent Design
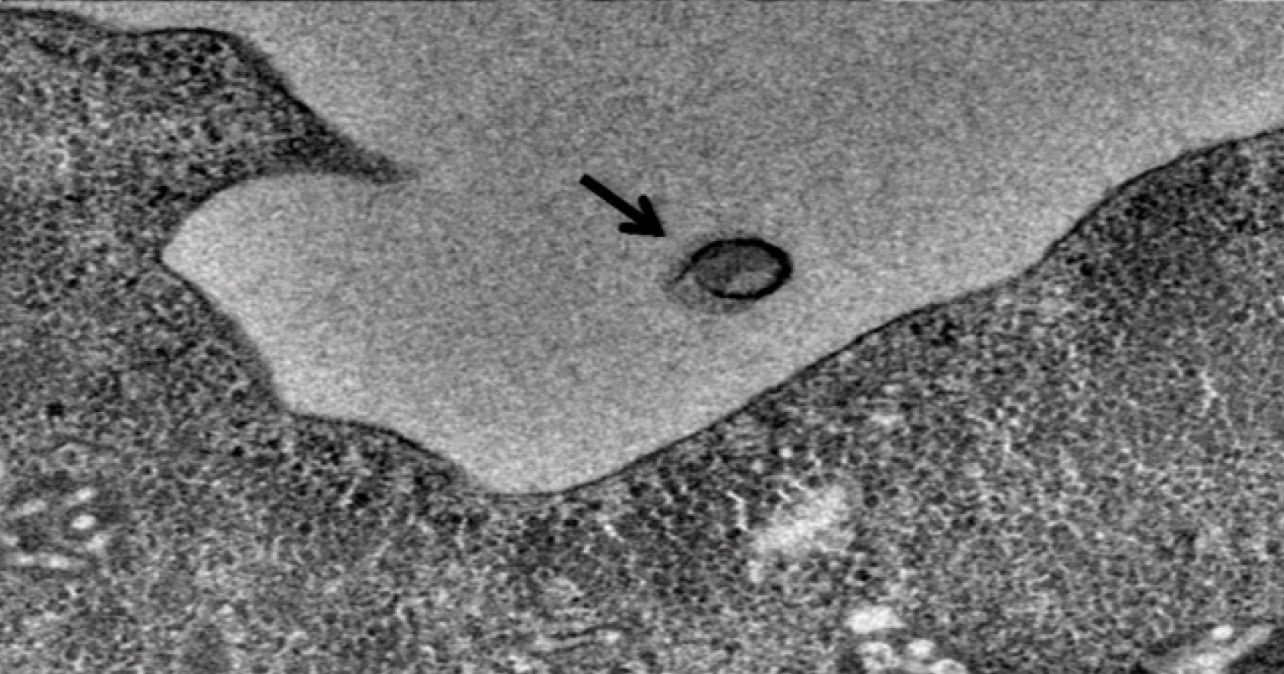
The actions of cells sometimes look sentient; no wonder we tend to anthropomorphize them. One system that exemplifies this purposeful action is the cell’s ability to sense harm going on in its interior. The cell puts up a flag on its outer membrane to signal any nearby phagocyte to come over and engulf it. It resembles an act of self-sacrifice for the greater good, like an altruistic soldier diving onto a grenade to save his comrades. Biochemists, with uncharacteristic humor, uniformly call this flag the “Eat Me!” signal.
News from Kyoto University exemplifies the point with the headline, “Eat me: The cell signal of death.” It begins with a cartoon of a lipid calling out to a phagocyte, “Eat me!” What is the mechanistic description for this phenomenon? The article says, “molecular mechanisms” are “involved in eliminating unwanted cells in the body.” The statistics are mind-blowing:
A nuclear protein fragment released into the cytoplasm activates a plasma membrane protein to display a lipid on the cell surface, signalling other cells to get rid of it. The findings were published in the journal Molecular Cell.
“Every day, ten billion cells die and are engulfed by blood cells called phagocytes. If this didn’t happen, dead cells would burst, triggering an auto-immune reaction,” explains iCeMS biochemist Jun Suzuki, who led the study. “It is important to understand how dead cells are eliminated as part of our body’s maintenance.” [Emphasis added.]
Flipping the Lipid Flag
Healthy cells come equipped with protein machines ready to scramble to raise the alarm flag. Biochemists, again with a sense of humor, call these proteins “scramblases” using the ubiquitous -ase suffix to indicate the machine’s substrate. The scramblase flips the lipid flag from the inside of the membrane to the outside of the membrane.
Wanting to learn more about this important maintenance operation, the research team at Kyoto found additional protein players involved. Some of the protein machines travel comparatively long distances like ambulances to a remote operations site:
Specifically, the scientists found that cell death signals lead to a nuclear protein, called XRCC4, getting cut by an enzyme. A fragment of XRCC4 leaves the nucleus, activating Xkr4, which forms a dimer: the linking of identical pieces into configurations. Both XRCC4 binding and dimer formation are necessary for Xkr4 to ultimately transfer lipids on the cell surface to alert phagocytes.
It’s a fail-safe operation, with protein parts acting like locks and keys. The mechanism ensures that the “Eat me” signal is not raised by mistake, leading to the death of a healthy cell. Moreover, Xkr4 is one of a squadron of scramblases that become activated quickly in cell death. The complexity of the system seems to keep growing. Is it any wonder there is no mention of evolution?
For more, see Masahiro Maruoka et al., “Caspase cleavage releases a nuclear protein fragment that stimulates phospholipid scrambling at the plasma membrane,” published in Molecular Cell.
Cannibalism in the Brain
The scramblase Xkr4 is highly expressed in the brain. Another mention of this phenomenon comes from the Salk Institute. The venerable lab, named after polio vaccine pioneer Jonas Salk, found something unexpected. The headline says, “In surprising twist, some Alzheimer’s plaques may be protective, not destructive.” Everyone thought the tangled plaques of amyloid protein in the brain were always nasty. It appears that the diseased plaques come from mis-regulation of a healthy process involving the “Eat Me” signaling system.
There are numerous forms of plaque, but the two most prevalent are characterized as “diffuse” and “dense-core.” Diffuse plaques are loosely organized, amorphous clouds. Dense-core plaques have a compact center surrounded by a halo. Scientists have generally believed that both types of plaque form spontaneously from excess production of a precursor molecule called amyloid precursor protein (APP).
But, according to the new study, it is actually microglia that form dense-core plaques from diffuse amyloid-beta fibrils, as part of their cellular cleanup.
Microglia cells used to be considered mere scaffolding in the brain. In the last decade or so, they have been recognized as essential players with many important functions. One of those roles is coming to light: cleanup of tangled protein clumps.
This builds on a 2016 discovery by the Lemke lab, which determined that when a brain cell dies, a fatty molecule flips from the inside to the outside of the cell, signaling, “I’m dead, eat me.” Microglia, via surface proteins called TAM receptors, then engulf, or “eat” the dead cell, with the help of an intermediary molecule called Gas6. Without TAM receptors and Gas6, microglia cannot connect to dead cells and consume them.
The team’s current work shows that it’s not only dead cells that exhibit the eat-me signal and Gas6: So do the amyloid plaques prevalent in Alzheimer’s disease. Using animal models, the researchers were able to demonstrate experimentally for the first time that microglia with TAM receptors eat amyloid plaques via the eat-me signal and Gas6. In mice engineered to lack TAM receptors, the microglia were unable to perform this function.
This discovery may lead to new targets for treatment of Alzheimer’s disease. Is this dreaded condition a result of a broken receptor on a cell designed to clean up dead amyloid proteins?
Once again, it appears that diseased states come from malfunction of engineered systems of molecular machines. Without the lipid “Eat Me” flag being hoisted to the cell surface by all the required accessory proteins — some of which travel all the way out from the nucleus to the membrane — dying cells would clutter the brain and other body tissues and lead to disease or death. Unsurprisingly, this article too has no use for evolutionary theory.
A Vast and Growing Catalog
The presence of the “Eat Me” signaling system adds to a vast and growing catalog of irreducibly complex systems necessary for life and health. Something had the foresight to see that this system would be needed. Something provided for all the parts to be present simultaneously, each part knowing its role. Foresight, as Marcos Eberlin convincingly shows in his book of that name, is a hallmark of intelligence.
