 Evolution
Evolution
 Intelligent Design
Intelligent Design
Non-Darwinian Adaptive Radiation Proposed
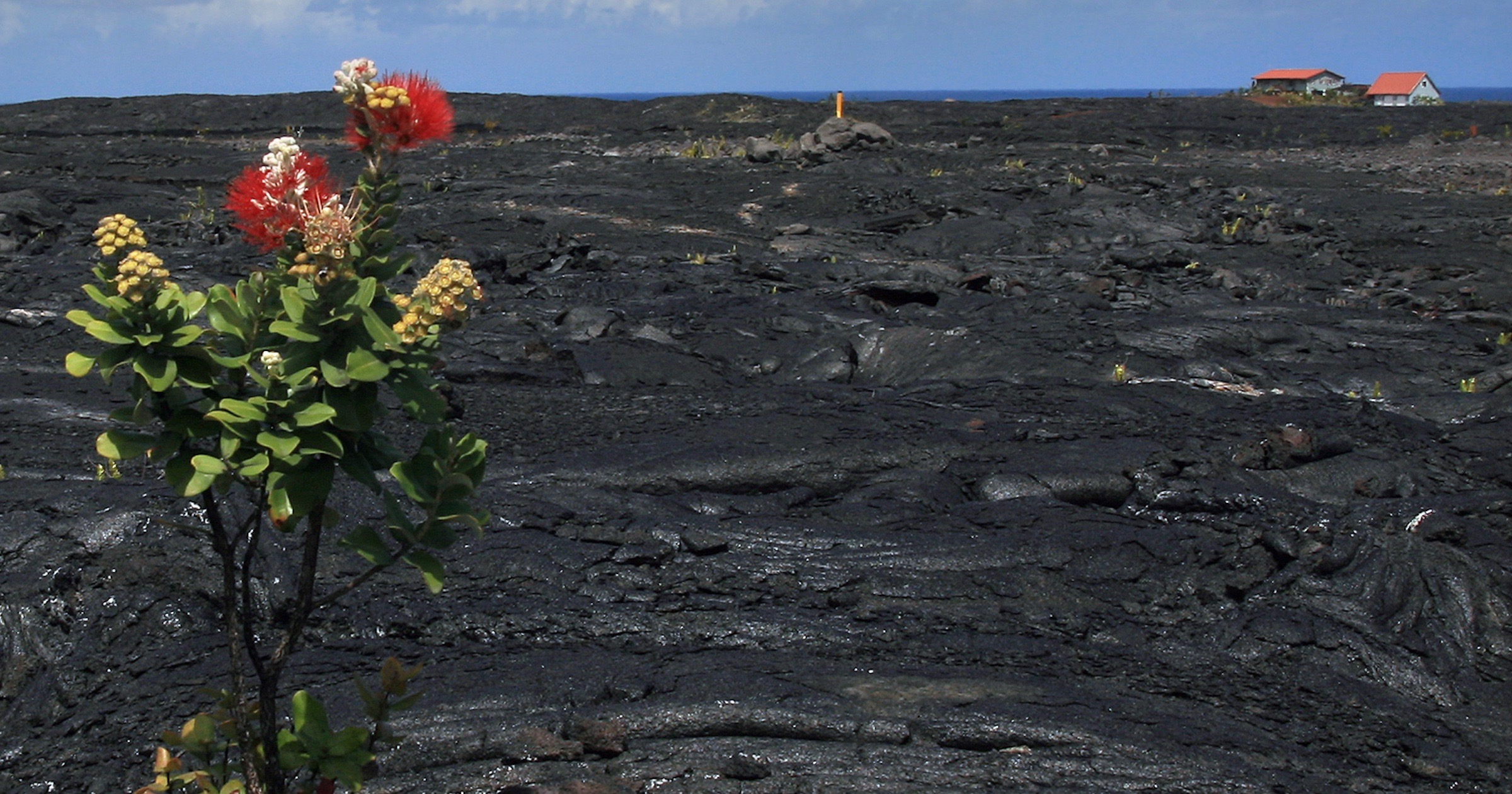
Design advocates have been pointing out the logical and evidential problems with “adaptive radiation” for many years (for example, see here for Casey Luskin’s comments). Darwinians insist that their theory can handle these cases of rapid evolutionary change — even the Cambrian explosion — even though Darwin himself preferred a theory of slow, gradual accumulation of small variations. His modern disciples say that the environment “forces” adaptations to occur rapidly sometimes but remain static other times. Surely some of them must have secret doubts about the special pleading put forth in spite of the evidence. For example, Amy McDermott in PNAS embeds evolutionary assumptions into a Darwinian narrative that pictures environments causing diversification:
He [Daniel Rabosky, University of Michigan] knew that bursts of rapid species formation often accompany transitions to new habitats. If fish arose in the oceans but then invaded freshwater multiple times, perhapsthey’d gone through bursts of speciation that explain the relatively high diversity of freshwater fish per unit area today. [Emphasis added.]
A habitat, however, has no foresight to cause a fish to adapt. It has no power to force rare beneficial mutations to appear that could be selected. Much less could it do this in “bursts” of speciation. The fish would easier go extinct unless something else is going on genetically.
Super-Abundant Code
Here’s a new idea to ponder. Choi et al., publishing in PNAS, have proposed a very un-Darwinian account of how “spectacular adaptive radiations” occur on oceanic islands such as Hawaii. This has been a “paradox of evolutionary biology,” they admit. Maybe the diversity is an outworking of “ancient polymorphisms” of ancestors with a rich gene pool —
Some of the most spectacular adaptive radiations of plants and animals occur on remote oceanic islands, yet such radiations are preceded by founding events that severely limit genetic variation. How genetically depauperate founder populations give rise to the spectacular phenotypic and ecological diversitycharacteristic of island adaptive radiations is not known. We generated genomic resources for Hawaiian Metrosideros — a hyper-variable adaptive radiation of woody taxa — for insights into the paradox of remote island radiations. We posit that divergent selection and differential sorting of an unexpectedly rich pool of ancestral variation drove the diversification of lineages. Recurring use of ancient variants from a richer-than-expected gene pool may explain how lineages can diversify to fill countless niches on remote islands.
There’s a hypothesis you may wish to consider. The genetic diversity was already present in the ancestors! Before now, evolutionists have assumed that an organism will have only the genes it needs to survive. If, instead, the ancestors had a “richer-than-expected gene pool” when they entered a new habitat, adaptive radiation amounts to a sorting out of existing information, not the creation of new information by neo-Darwinism’s mutation/selection process.
A large pool of ancestral variants would have served as readily available genetic variation for adaptation, without the waiting times required for de novo adaptive mutations. Indeed, we discovered that genomic divergence (and potentially the genetic basis of reproductive isolation) between early diverging Metrosideros taxa was shaped by divergent selection targeting ancestral variations over evolutionary young de novo variations.
Design and Foresight
It would be reasonable to expect an intelligent designer to have the foresight to do this. If the goal was to fill the earth with living creatures able to adjust to a wide variety of environments, the solution would be to enrich the operating system of each type with a rich pool of code and apps for handling unforeseen challenges — more than would be needed for the initial launch. Darwinism, by contrast, would not expect to keep code around that was not being used.
It should be stated that the authors are not denying Darwinism. “Adaptive radiations demonstrate the remarkable power of natural selection as a driver of biological diversity,” they say. Their attention is on islands, which they believe to be a special case:
Adaptive radiations on remote oceanic islands are especially interesting, as colonization of remote islands is expected to involve population bottlenecks that restrict genetic variation. Adaptive radiations in such settings are especially impressive and even paradoxical, given the generation of high species richness from an initially limited gene pool. Several classic examples of adaptive radiation occur on oceanic islands, such as Darwin’s finches from the Galapagos islands, anole lizards from the Caribbean islands, Hawaiian Drosophilids, and Hawaiian silverswords, to name a few.
The authors chose a plant because “There are no genomics studies of plant adaptive radiations in geographically restricted systems such as remote islands.” The example they researched is a woody plant in the Hawaiian islands, Metrosideros, that exhibits considerable diversity after what they believe was a single founder event.
Hawaiian Metrosideros is a landscape-dominant, hypervariable, and highly dispersible group of long-lived (possibly >650 y) woody taxa that are nonrandomly distributed across Hawaii’s heterogeneous landscape, including cooled lava flows, wet forests and bogs, subalpine zones, and riparian zones. About 25 taxa or morphotypes are distinguished by vegetative characters ranging from prostate plants that flower a few centimeters above ground to 30-m-tall trees, and leaves range dramatically in size, shape, pubescence, color, and rugosity.
Faced with the paradox of the “founder effect” in traditional neo-Darwinism, which should have caused inbreeding and genetic decline instead of adaptive radiation, they took a different approach. “Our findings suggest that diversification of Hawaiian Metrosideros was facilitated by reassortment of an unexpectedly rich pool of ancestral polymorphisms.”
Considering Other Hypotheses
They discounted other hypotheses. They knew that a single founder with a limited genome should degenerate due to inbreeding. If there were several founders, however, offering more standing variation, hybridization and introgression could only go so far. Did multiple species arrive in Hawaii independently? Unlikely, they say, because of the distance from the presumed ancestral home in Australia or its closest transfer point in the Marquesas Islands south of the equator, 3,000 km away. They consider polyploidy as a source of genetic variability, but “it is not certain how much of the radiation can be attributed to the duplication of functional genetic elements or to the ploidy increase itself.”
Nor is the variability simply a case of phenotypic plasticity. Experiments on heritable traits indicate that the morphotypes are distinct genetic groups. Genetic comparisons with specimens from New Zealand and other Pacific islands support their remaining hypothesis: the ancestral stock was already rich with genetic diversity. Phylogenetic analysis produced a reticulate network diagram, not a branching tree, probably due to subsequent hybridization of the diversifying populations.
After all their genomic analysis of 131 individuals from 11 taxa found across the islands, they decided, “Our findings suggest that diversification of Hawaiian Metrosideros was facilitated by reassortment of an unexpectedly rich pool of ancestral polymorphisms.” They believe their conclusion can shed light on other cases, like the African cichlid fish and Darwin’s finches. New functional mutations did not just pop into existence by chance; there was “considerable ancestral polymorphism” in the gene pool that was able to be sorted in various ways.
Lego Diversification
Speaking of cichlids, McDermott entertains a similar non-Darwinian explanation for the rapid diversification of these Darwin-icon fish in African lakes. She quotes evolutionary biologist Ole Seehausen from the University of Bern, whose team found similar genomic richness in those populations.
They found hundreds of distinct DNA regions strongly tied to different ecological niches and scattered across 22 chromosomes. “We think that’s the key to make hundreds of species and not just two or three,” Seehausen says. When the fish hybridize, they can rearrange these modular genes, “almost like Lego bricks,” he says, to build many possible combinations suited, for example, to a rocky inshore fish that feeds on insects, or one that eats the same bugs but lives in weedy lake grass.
Explaining diversity by ancestral genomic richness relieves biologists of having to claim that “500 new species evolved in just the last 15,000 years” by the old neo-Darwinian process of waiting for beneficial mutations to show up. It might also relieve them of having to wave the magic wand of “convergent evolution” if ancestral genomic richness equipped organisms to adapt. The case of convergence in marine mammals — see Yuan et al. in PNAS — could be reconsidered with that in mind. And another rethink could come for “junk DNA” that has misled evolutionists for decades. Researchers at the Whitehead Institute at MIT are looking at non-coding regions as locations where adaptation is occurring. They’re calling it “The Origin of Species: DNA Edition” which is very different from Darwin’s conception. The light went on for researchers when they realized that the difference between apes and humans, for instance, may reside in the non-coding regions that had been dismissed as junk:
“After we realized the function [of satellite DNA in the cell], the fact that satellite DNA is quite different between species really hit like lightning,” Yamashita said. “All of a sudden, it became a completely different investigation.”
Follow-Up Questions
The conclusion by Choi et al. about ancestral genomic richness could be expanded by researchers not already sold on neo-Darwinism. ID-friendly geneticists could look for additional examples of this explanation for spectacular radiations in biology. The distribution of super-abundant functional information implies top-down “overdesign” rather than bottom-up emergence. What a great starting point for taking the speciation problem out of naturalism and into intelligent design.
Specifically, some follow-up questions for design research might be as follows:
- What are the limits of adaptation by ancestral genome richness? (See Brian Miller’s comments here.)
- How does this differ from “front-loading” to get humans from bacteria, as some theistic evolutionists argue? (Suggested approach: which one is testable as opposed to philosophical?)
- What parts of non-coding regions contain functional information for adaptation?
- Does the genome contain algorithms for reprogramming non-coding regions (i.e., transcription factors) in response to environmental cues?
- What other sources of information can organisms access? (For example, “Borg” libraries for bacteria, discussed here, retroviruses, transposable elements, hybridization and introgression.)
- How can Darwin skeptics turn these concepts into research projects that all biologists would consider worthwhile?
