 Evolution
Evolution
 Intelligent Design
Intelligent Design
Third Paper Presenting an Engineering Analysis of the Flagellum Makes the Case for Intelligent Design

Over this past year I’ve covered two papers by engineer Waldean Schulz on the engineering design of the bacterial flagellum (see my reviews of his first paper and his second). Now, as Brian Miller noted earlier, Schulz has published his third and final peer-reviewed paper on the topic in BIO-Complexity, titled “An Engineering Perspective on the Bacterial Flagellum: Part 3 — Observations.” He concludes that a system as complex as the flagellum “seems profoundly unlikely to naturally evolve in the absence of foresight and mindful intent.”
Sketching the Relationship Between Flagellar Components
Schulz begins by listing the various parts of the flagellum, detailing their functional-mechanical-biochemical relationships. See Figure 1 from the paper below:
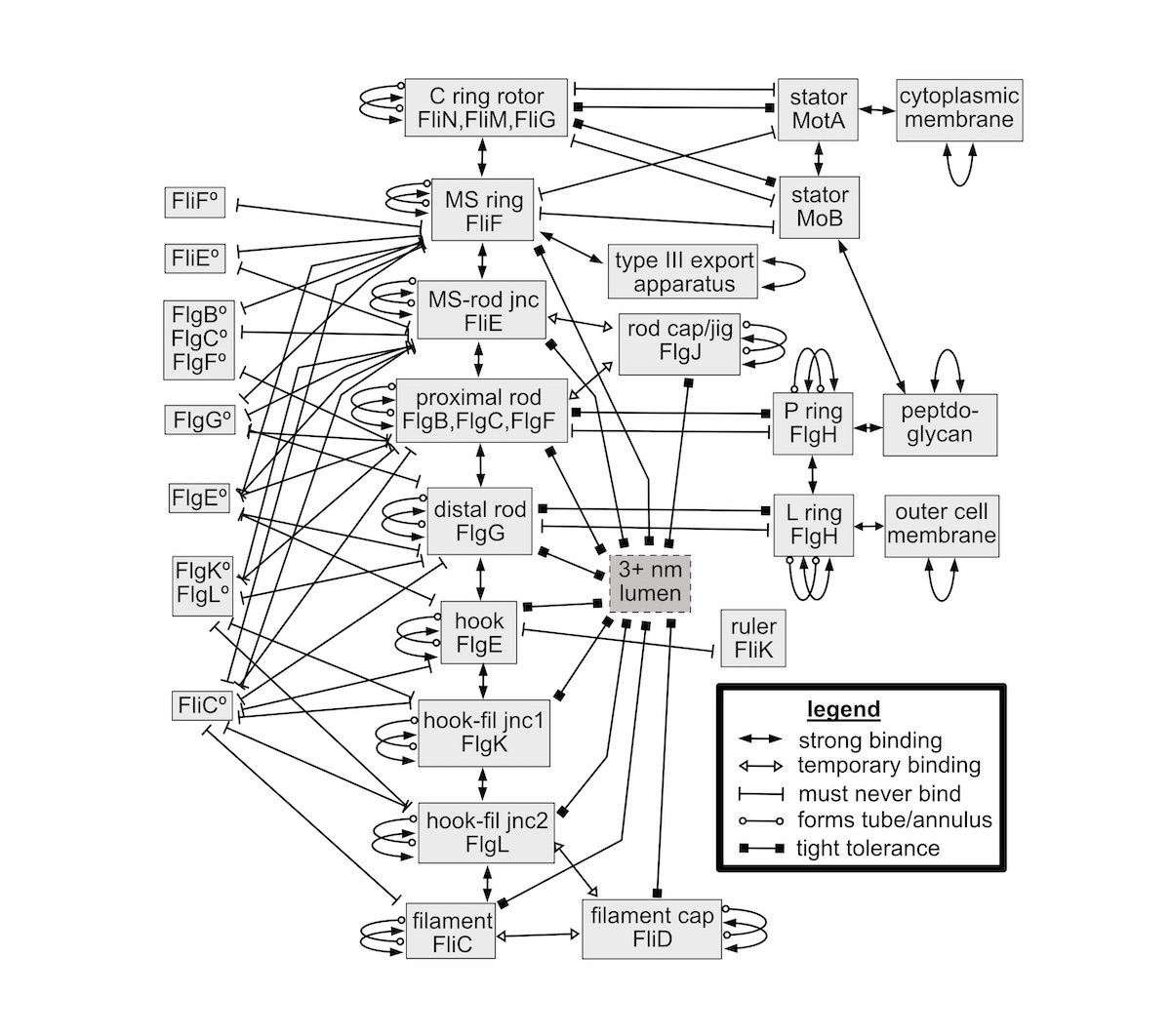
This diagram — showing interactions in terms of engineering schematics — is unlike any description of the flagellum that I’ve seen in any other technical paper. It implies a whole new level of complexity. Not just the necessary mechanical parts must be present but they must be designed to interact in a chemically and mechanically coordinated manner that allows the molecular machine to function.
Six Specifications for Flagellar Components
Here’s an example of one type of specification:
The lines in Figure 1 represent highly specific properties of the proteins composing the various subassemblies. First, the molecular structure of the proteins forming an annulus or tube must have the property that the proteins have binding sites which firmly connect the proteins so they sequentially abut. In doing so, each protein must effectively form an N-degree arc. That is a very specific geometrical and amino acid sequence property for a folded protein, which only extremely rare protein configurations could meet. Further, for proteins forming a tube, each round of the helical, coiled-rope-like, end-to-end connected proteins, and each loop of the helix binds to the preceding and succeeding loops to form a stable tube, and thus can efficiently transmit torque by being tangentially rigid. In other words, many of the proteins must bind to four other adjoining proteins: the proteins fore and aft along each loop of the helix, and the proteins of the preceding proximal and succeeding loops around the helix. The neighboring proteins may be similar (such as within the filament) or dissimilar (where one subassembly binds to the next). That is, the proteins of each subassembly must firmly bind with the proteins of at least one other subassembly. These binding properties require rigorous, very specific requirements on the folded chain of amino acid residues of those proteins, that is, the aligned locations of non-covalent binding between two similar or dissimilar proteins.
This is just the first of a list of six specifications that flagellar proteins must meet — the others being (2) flexibility, (3) temporary binding relationships, (4) non-attractiveness to particular components, (5) size tolerances, and (6) temporal accuracy of final bonding relationships (i.e., binding at the right time). To elaborate on specification (4), Schulz explains that this has important mechanical implications:
[C]ertain pairs of subassemblies must have no attraction as shown by the “must never bind” lines in Figure 1. For example, the proximal rod must freely rotate inside the P ring. That property strictly limits the amino acid configuration in the proteins involved. It further implies that the rod must be nearly circular, and so must the “donut hole” of the P ring (in cross-section) through which the rod fits with very small tolerance. While the proximal rod must rotate with little friction, the P ring and proximal rod nevertheless must be so close to each other that there is no “leakage” through the space between them. Similar observations hold for the L ring and distal rod and for the stator and rotor subassemblies. That is, the inner diameters of the “donut hole” of the rings must very closely match the outer diameters of the rod subassemblies. The circularity requirements and the tight tolerances are yet two more geometrical properties needing to be met by the constituent proteins and the way they self-coalesce.
These six specifications “must all be present, so the already extremely rare protein configurations of the first property are even more rigorously restricted by the other required properties.” This implies a form of irreducible and/or specified complexity where a core number of parts must not only be present but must meet the proper specifications (material, mechanical, biochemical, etc.) in order to yield a functional flagellum.
Tying It Together
Schulz also ties together the “top-down specification” requirements identified in his first paper with the “bottom-up” implementation observed in his second paper. Here’s one example, representing part of Table 2 of the paper:
Table 2: Comparison of top-down specification with bottom-up analysis: Propulsion
| top-down specification | bottom-up analysis |
| required propulsion functions | archetypical implementation |
| for all schemata | (see below) |
| power source | ion (H+ or sodium) gradient |
| power-to-motion means | rotary electric motor |
| external component(s) | rod end, hook, filament |
| foundation/substrate | inner/outer membranes, peptidoglycan |
| rotary schema: rotary subsystem | (see below) |
| armature | MS ring: FliF proteins |
| motor rotor | C ring: FliG, FliM, FliN |
| Shaft | rod: FlgB, FlgC, FlgF; FlgG |
| (axial bend, if side mount) | hook: FlgE |
| helical propeller | filament: FliC, FliD (cap) |
| (possible adaptors) | hook-filament: FliE, FlgK, FlgL, FlgM |
| seals-bearings | P ring & L ring: FlgI, FlgH |
| motor stator | MotA, MotB |
Schulz offers a profound insight: each constraint that was identified in the top-down analysis has a corresponding part that was observed in the bottom-up analysis: “Clearly there is high correlation between the top-down and bottom-up perspective. What is the implication of this correlation? It suggests the configuration of the flagellum is purposeful.”
Diverse Perspectives on the Flagellum
Schulz then presents unique observations about the flagellum from widely different perspectives: that of an inventor, an engineer, a molecular biologist, an evolutionist, and a philosopher.
As an experienced inventor, Schulz observes that “many patents have been awarded simply for novel proteins. Yet the design of a single protein involves far less intellectual content and originality than would be required to design a coherent complex of proteins self-assembling into an organelle. This exposes a certain irony: the intellectual input of inventors is recognized in a human-designed protein but not in a natural protein — or in a coherent subsystem of proteins composing an organelle like the flagellum.” This calls to mind a paradox I’ve long observed: our legal system is well-situated to detect design and does so every time a person is convicted of a crime, yet science often resists making design inferences.
This irony or paradox must be resolved. But how can we recognize design in the flagellum? The engineer’s perspective helps address this question:
[A]n experienced engineer would fully appreciate the mental effort, insightful creativity, inventive genius, and foresight that even a rather simple device requires. It begins with observing a need or problem, implying purposeful insight. That is followed by identifying the available resources (materials, tools, existing parts), necessary functions, normal environment, physical constraints, and so on. Then such factual input is followed by one or more design schemata. While numerous design options may be conceived, a very few fully comply with the foregoing requirements and constraints. This whole process requires significant mental effort and is far from trivial or accidental. Nevertheless, all that abstract specification still does not instantiate a physical entity.
What is needed is foresight and planning to convert the abstract specification into physical reality — the very process of intelligent design. Here’s how engineers do it, according to Schulz:
A series of one or more prototypes must be physically constructed. A prototype is then tested for compliance for substantially satisfying the need or solving the original problem and, more specifically, all the stated and logically derived requirements. All that applies to the flagellum, as the foregoing discussion has shown.
And what is the best method to find the solution to a problem? Is it a blind trial and error search? Schulz points out that as an inventor Thomas Edison used many different materials when trying to construct a light bulb — but they were all intelligently chosen based upon their properties. Similarly, he observes that engineers at “Microsoft, Apple, or Google would never — could never — produce specified software by any blind search.” Inventors and engineers appreciate that intelligence is a far more efficient search process than blind trial and error.
He also compares the process of testing different designs to the operation of natural selection. Just as testing can validate or invalidate a given design, selection can preserve or “select out” a given feature based upon whether or not it provides an advantage. But Schulz notes that selection is merely a “passive filter” — it cannot create a new design. Thus, “What seems incredible is that mindless, random mutations could ever innovate and instantiate any coherent, intricate functionality beyond trivial modifications to existing functionality.” He notes that the “odds are massively against” a protein meeting the specifications he listed and forming “an interacting complex of new proteins which properly bind together, or even simply one custom, functional, folded protein.” Those specifications also count against co-option, because this model “presumes that such proteins possess the bevy of characteristics … such as matching binding sites, not binding to each other in many cases, precise dimensions relative to each other, coordinated functionality, control of fabrication and assembly order, and appropriate stoichiometry.” In other words, the whole idea of co-option — to take a protein, and retool it to perform some new entirely function — implies that a protein must be modified and remade to meet a totally new set of specifications. This suggests that co-option explanations must overcome a higher bar than has often been appreciated.
The molecular biologist is less concerned with origins than with how systems work. Schulz envisions the molecular biologist observing that “each of the structural proteins of each of the flagellum’s subassemblies requires an extremely precise molecular configuration in order to simultaneously comply with several very specific required properties.” Schulz then gives new perspectives on some of the constraints that flagellar proteins must meet:
- Binding strength to overcome molecular forces: “the copies of the constituent protein(s) of a subassembly must bind tightly to themselves, because at this scale, where Brownian turbulence is dominant, the flagellum must be exceedingly tough and robust and must efficiently transfer torque”
- Proper folded geometry to produce overall shape: “the folded geometry of many of the proteins must be curved arcs that lead to the formation of annuli and tubes — binding head-to-tail; those proteins forming tubes must also bind to like proteins in preceding and succeeding turns of the helix”
- Matching of proper parts for protein-protein mechanical interactions: “the diameters of the annuli and tubes are critically matched, to form a seal and yet to allow efficient rotation”
- Binding to neighbors: “each copy of the constituent proteins of a subassembly must tightly bind to the proteins of the one or two adjacent subassemblies”
As for the observations of an evolutionist, Schulz quotes Aizawa (2009) admitting — from an evolutionary perspective — the beautiful design of the flagellum:
Since the flagellum is so well designed and beautifully constructed by an ordered assembly pathway, even I, who am not a creationist, get an awe-inspiring feeling from its ‘divine’ beauty…. However, if the flagellum has evolved from a primitive form, where are the remnants of its ancestor? Why don’t we see any intermediate or simpler forms of flagella than what they are today? How was it possible that the flagella have evolved without leaving traces in history?
Yet Aizawa says the flagellum “has been streamlined by evolution to minimize the time of the assembly process” and “has acquired its beauty by evolving…” How can we resolve his admission that the flagellum is “is so well designed and beautifully constructed” that it induces an “awe-inspiring feeling from its ‘divine’ beauty,” with his statement that the flagellum evolved? Now writing as a philosopher, Schulz gives a simple answer: Aizawa’s contradictory statements stem from his “philosophical prior commitment to Naturalism.”
Evolutionary Obstacles and Intelligent Design
Schulz provides more commentary from an evolutionary perspective. He notes that natural selection can optimizefeatures but not innovate new ones. Without intelligent guidance, blind selection often gets stuck on local fitness peaks and cannot always traverse to highly adapted features sitting on other adaptive peaks: “without intelligently chosen, appropriate starting values — or a more sophisticated, robust algorithm — simpler algorithms often converge to local, suboptimal values.”
He further quotes Merino (2009) making the crucial observation that “Comparison of the complete genome sequences of flagellated bacteria revealed that flagellar structural proteins are based on an ancient core set of 24 flagella genes that were present in the common ancestor to all Bacteria.” In other words, there seems to be an irreducibly complex core of protein components common to all bacteria and these pose a severe challenge to evolutionary explanations of the origin of the flagellum. Schulz appreciates the implications of this irreducibility of the flagellar system:
Two dozen genes require quite a few innovative origins lacking detailed explanations — origins presumably occurring nearly simultaneously. Further, is there any evidence that proteins produced by a smaller combination of those genes have any function? In any case, as noted above, there is yet no trace of flagellar lineage from some simpler, functional motile organelle.” This poses a challenge to the evolutionary perspective. “The future work,” Schulz writes, for any “evolutionary theorist, is (1) to provide a detailed hypothesis for how all the tightly constrained interlocking coherence described above could have evolved naturalistically under real-world constraints and (2) to show evidence that such a scenario actually transpired in the past” and “hypothesize some putative detailed, step-by-step scenario to explain how the flagellum and its control system was blindly engineered naturalistically.
Schulz is doubtful that blind engineering is possible — but he does not pretend that all questions have been answered:
How would portions of a nascent flagellum be protected from degradation while the remainder were yet to be gradually evolved? If some of the requirements discussed above could be omitted, what function would that provide? These are real questions that demand answers. Yes, these are hard questions, and we surely do not know nearly enough yet to answer them. The challenge is to answer them.
Importantly, the fact that there is still much to learn about the flagellum is not evidence that we cannot consider intelligent design. In fact, based upon what we do know, Schulz argues “it seems disingenuous to pretend that questions about intelligent causation are irrelevant and inconsequential when so much is already known about the hierarchical assembly, control, and function of the flagellum.” He concludes: “A motility organelle of this scope and scale seems profoundly unlikely to naturally evolve in the absence of foresight and mindful intent.”
