 Evolution
Evolution
 Intelligent Design
Intelligent Design
 Life Sciences
Life Sciences
If Nanomotors Are Designed, Why Not Biomotors?
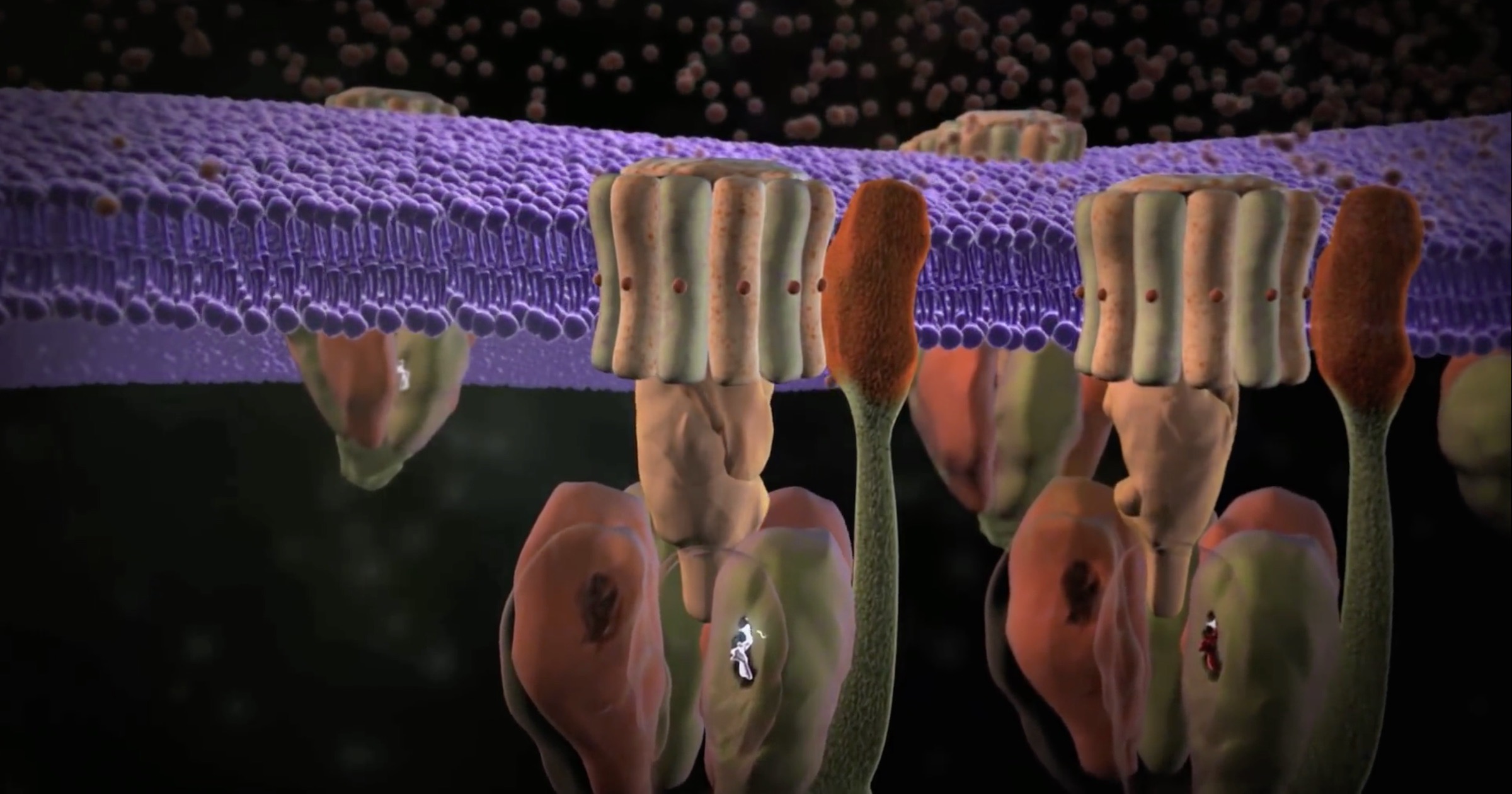
If engineers know how much effort goes into making an object spin that is just a few nanometers wide, one would think they would stand in awe of biomolecular machines that do much more — machines that perform functions and are linked into signal transduction pathways and can reproduce themselves. Wouldn’t it be a refreshing change to have them admit that biological motors look intelligently designed?
Watch the new nanomotor built by engineers at the University of Texas at Austin. It goes around, and around… and around.
The new motor is less than 100 nanometers wide, and it can rotate on a solid substrate under light illumination. It can serve as a fuel-free and gear-free engine to convert light into mechanical energy for various solid-state micro-/nano-electro-mechanical systems. [Emphasis added.]
ATP synthase, though, is almost an order of magnitude smaller, and it does much more than rotate. It turns a crankshaft that builds three ATP molecules per revolution, running on protons. It is anchored to the mitochondrial membrane in animals and the chloroplast membrane in plants. A plant’s “fuel free” nanomachines run on light, too. And Brownian motion doesn’t slow it down, like the UT engineers had to worry about.
“Nanomotors help us to precisely control the nanoworld and make up new things we want for our real world.” said Jingang Li, a PhD graduate from Zheng’s group and the lead author of this study.
Biological machines are part of the real world, aren’t they? Is Dr Jingang Li aware that trillions of rotary engines are spinning in his own body as he speaks? The publicist does give a little credit to biology:
The reason scientists are so enamored with creating these tiny motors is because they mimic some of the most important biological structures. In nature, these motors drive the division of cells and help them move. They combine to help organisms move.
OK. But earlier, Jinang’s associate professor said, “Life started in the water and eventually moved on land” — presumably all by itself. If the UT team really wanted to mimic biological machines, why not toss some chemical elements in water and wait for a few billion years for it to move on land?
Cheap Imitation
In New Scientist, a reporter boasts that a “Tiny nanoturbine is an autonomous machine smaller than most bacteria.” Credit is given to a rotating enzyme (presumably ATP synthase) for the inspiration:
Cees Dekker at Delft University of Technology in the Netherlands and his colleagues created the turbine after being inspired by a rotating enzyme that helps catalyse energy-storing molecules in our cells. They wanted to build a molecular machine that could similarly do work, like adding energy to biological processes or moving other molecules, without having to be repeatedly pushed or manipulated in some way.
Their little nanoturbine, just 25 nm in diameter can extract energy from salt water and rotate at 10 rpm. The article doesn’t mention that ATP synthase is half that size and runs at up to 6,000 rpm, without the problems of random thermal fluctuations that make their nanoturbine difficult to control.
“This is not that different than an engine you have in your car,” says Dekker. “You put in gasoline, you get mechanical work. With the nanoturbine, you add the salt mixture, you get mechanical work, namely rotations.” The researchers also found that they could power the turbine by exposing it to electric voltage or having flowing water turn it much like wind turns a windmill.
These structural chemists surely know that cars are intelligently designed. Why is there hesitancy to say that superior engineering design is found in the biological motors that inspired them?
Better than Nature?
News from the University of California, Riverside, claims to have bested nature. Scientists there say that they have built “artificial photosynthesis” that could be much more efficient at improving crop yields than biological photosynthesis:
Photosynthesis has evolved in plants for millions of years to turn water, carbon dioxide, and the energy from sunlight into plant biomass and the foods we eat. This process, however, is very inefficient, with only about 1% of the energy found in sunlight ending up in the plant. Scientists at UC Riverside and the University of Delaware have found a way to bypass the need for biological photosynthesis altogether and create food independent of sunlight by using artificial photosynthesis.
The way that’s worded, it sounds like they just stumbled on “a way” to improve on nature. A look at the Methods section of their paper in Nature Foods, though, shows a highly intricate procedure for preparing the setup: anodes, cathodes, flow electrolyzer, and other parts using multiple elements in precisely arranged ways. Even so, their system only makes acetate (C2H3O2) — a relatively simple compound — nothing like the complex carbohydrates made by plants. If certain plants can use acetate to grow their complex molecules without photosynthesis, fine; but that’s a far cry from what plants do on their own.
The researchers admit their device was “engineered.” It may find application in places where crop plants are hard to grow, such as on a spacecraft. But growing the food will require the elaborate biochemistry in plant machines; it will not work on electrolysis alone. To fulfill their boast, now let the engineers code molecules that will build their devices from soil and deliver acetate to food plants automatically and in the right proportions. Then let them engineer a way to package the code in seeds.
Information Please
Researchers at the Howard Hughes Medical Institute have created a “DNA Typewriter” that “taps out a record inside cells.” It allows them to store messages in DNA code.
While developing a new system for recording within cells, geneticist Jay Shendure and his team decided to give it a test run by using it to encode text. Since their invention relied on a nearly brand-new recording medium, DNA, they wanted to use messages that evoked a sense of historical significance.
Two choices were obvious: “What hath God wrought?,” a Biblical quote used by Samuel Morse in the first long-distance telegraph transmission, and the more mundane, “Mr. Watson, come here!” spoken by Alexander Graham Bell to his assistant in the first telephone call.
A line from Dickens was also considered, but a Korean member won by using a line from a K-Pop song. The team hopes to use their technique to genetically engineer cells that can barcode individual cells and store records of the cell’s activity. But doesn’t the sequence of letters in natural DNA qualify as a text?
Rightly So
In each of these examples, molecular engineers showed great pride in their achievements, and rightly so. They considered how they might be used for the good of mankind. Not one of them made the most logical inference, though, that the very biological cells that inspired their work were also engineered by design. Maybe some day soon they will not be ashamed to say so. Many great scientists used to proclaim that without hesitation.
