 Evolution
Evolution
 Intelligent Design
Intelligent Design
Game Over? Nick Lane Wants Another Inning
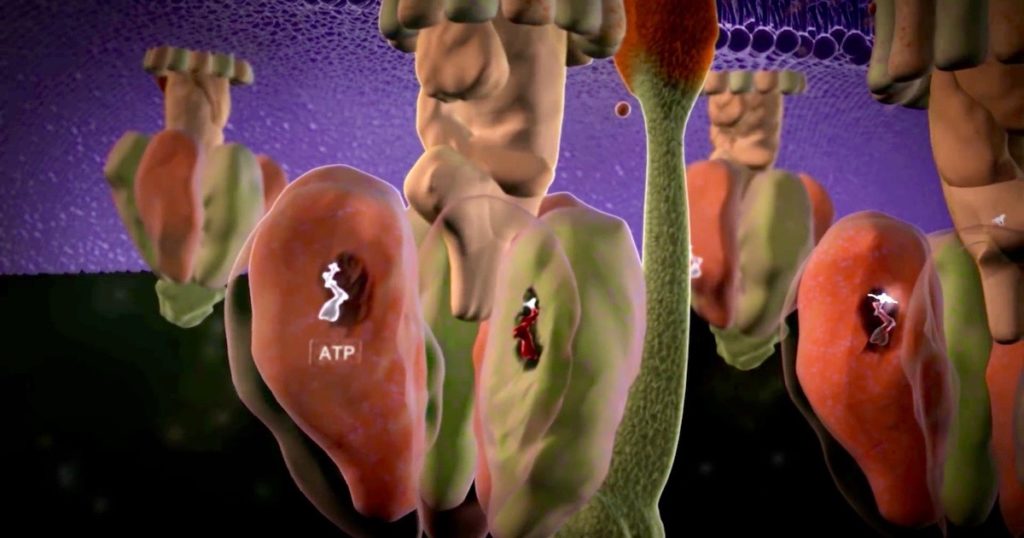
The field of origin of life research has struck out at the bottom of the ninth. The crowds file out of the stands. Suddenly, eight players run onto the field! “Wait! Wait!” they cry. “Let us have a time at bat!”
The rescue team, led by Nick Lane of University College London, waves a paper over their heads. It’s hot off the press from PLOS Biology, titled, “A prebiotic basis for ATP as the universal energy currency.” Lane shouts, We have the intermediate! It’s AcP! One of the refs eyeballs the paper for a minute. Will it be worth calling the teams back onto the field for another inning?
A Plausible Scenario?
The gist of the hypothesis is that acetyl phosphate (AcP), a simple molecule with the formula C2H5O5P, can phosphorylate ADP into ATP in water, if ferric ion (Fe3+) is present. The team believes their lab work offers a plausible scenario for prebiotic ATP formation without the need for ATP synthase.
ATP is universally conserved as the principal energy currency in cells, driving metabolism through phosphorylation and condensation reactions. Such deep conservation suggests that ATP arose at an early stage of biochemical evolution. Yet purine synthesis requires 6 phosphorylation steps linked to ATP hydrolysis.This autocatalytic requirement for ATP to synthesize ATP implies the need for an earlier prebiotic ATP equivalent, which could drive protometabolism before purine synthesis. Why this early phosphorylating agent was replaced, and specifically with ATP rather than other nucleoside triphosphates, remains a mystery. Here, we show that the deep conservation of ATP might reflect its prebiotic chemistry in relation to another universally conserved intermediate, acetyl phosphate (AcP), which bridges between thioester and phosphate metabolism by linking acetyl CoA to the substrate-level phosphorylation of ADP. We confirm earlier results showing that AcP can phosphorylate ADP to ATP at nearly 20% yield in water in the presence of Fe3+ions. We then show that Fe3+ and AcP are surprisingly favoured. [Emphasis added.]
Sounds Impressive. Can It Work?
The team tells the referee about additional surprising benefits of their intermediate. Visions of the Miller spark apparatus come to mind:
Surprisingly, our results demonstrate that maximal ATP synthesis occurred at high water activity and low ion concentrations, indicating that prebiotic ATP synthesis would be most feasible in freshwater systems.Likewise, ferrous iron can be oxidized to ferric iron by photochemical reactions or oxidants such as NO derived from volcanic emissions, meteorite impacts, or lightning strikes, which also points to terrestrial geothermal systems as a plausible environment for aqueous ATP synthesis.
Questions & Answers
What about hydrothermal vents?, the referee asks. Aren’t those the preferred locations for prebiotic environments? “[O]ur results do not exclude submarine hydrothermal systems as potential environments for this chemistry,” they beam with pleasure. But it couldn’t happen today, they explain, because “high concentrations of Mg2+ (50 mM) and Ca2+ (10 mM) precluded ATP synthesis, implying that this chemistry would not be favoured in modern oceans.” The referee, frowning a bit, senses some special pleading going on.
Other referees walk up to see what the commotion is about. After listening in, they start asking questions.
Did you try this in a natural setting? No, we bought chemicals from Fischer and from Sigma-Aldrich, and then mixed them in our lab under controlled conditions. (See the Materials and Methods section.)
How did you get the ingredients to link up? We used store-bought catalysts and mixed them with store-bought nucleotides and phosphorylating agents. Then we shook them and heated them.
Why do you think that represents a plausible prebiotic environment? “AcP is unique among a panel of relevant phosphorylating agents in that it can phosphorylate ADP to ATP, in water, in the presence of Fe3+. AcP is formed readily through prebiotic chemistry and remains central to prokaryotic metabolism, making it the most plausible precursor to ATP as a biochemical phosphorylator.”
Are you likely to find sufficient concentrations of AcP and ferric ion in natural water conditions for this to have happened on the early earth? Uh, we didn’t test that.
Wait a second; adenosine is a nucleoside base that includes ribose. How did that form in water? That is a problem, we agree.
Did you test for chirality? Uh, no.
Did you come up with a plausible container to hold the ATP? That was not part of our investigation, no.
OK, so you get some ATP under special conditions. ATP has a half-life of under 5 minutes in water. Do you expect it to hang around long enough to be useful in some protocell? We did not think about that in this paper, no.
ATP is not alive, obviously. What would happen next? Presumably some primitive metabolic process could utilize it for energy.
Like what? “Recent experimental work shows that the core of autotrophic metabolism can occur spontaneously in the absence of genes and enzymes. This includes nonenzymatic equivalents of the acetyl CoA pathway and parts of the reverse Krebs cycle, glycolysis and the pentose phosphate pathway, gluconeogenesis, and amino acid biosynthesis. Recent work demonstrates that some nucleobases can also be formed following the universally conserved biosynthetic pathways, using transition metal ions as catalysts. The idea that ATP could have arisen as a product of protometabolism starting from H2 and CO2 is therefore not unreasonable….”
What, exactly, is “protometabolism”? Does it have any meaning outside of a living context? (Silent stares.)
Who decides what is reasonable? I guess we do.
The paper says that “biological purine synthesis specifically involves 6 phosphorylation steps that are catalysed by ATP in modern cells.” Adenine is a purine. How do you get past the chicken-and-egg problem of needing ATP to make ATP?“If ATP was indeed formed in a monomer word via a biomimetic protometabolism, then an earlier ATP equivalent must have driven the phosphorylation steps in purine synthesis.”
Can you describe a plausible earlier ATP equivalent? Actually, “A major question for prebiotic chemistry is how could an energy currency power work” if not ATP.
And how did ATP come to replace it, whatever it was? “Why this early phosphorylating agent was replaced, and specifically with ATP rather than other nucleoside triphosphates, remains a mystery.”
So how did your simple ATP-generating process get replaced by ATP synthase? Well, it is well known that “the ATP synthase powers a disequilibrium in the ratio of ADP to ATP, which amounts to 10 orders of magnitude from equilibrium in the cytosol of modern cells. Molecular engines such as the ATP synthase use ratchet-like mechanical mechanisms to convert environmental redox disequilibria into a highly skewed ratio of ADP to ATP.” But we cannot say how that happened.
But how could a simple prebiotic system composed mostly of monomers sustain a disequilibrium in ATP to ADP ratio that powers work? Well, “One possibility is that dynamic environments could sustain critical disequilibria across short distances such as protocell membranes.”
Didn’t you just assume the existence of a protocell with a membrane? Where did those come from? Look, we’re not trying to come up with a complete picture of how life originated. We’re just trying to explain why ATP is the universal energy currency for life as it exists today, and how it might have emerged.
Emerged… by chance, you mean? Isn’t that circular reasoning? How so? What other possibility is there?
There’s intelligence, the only cause ever observed that is capable of assembling complex parts into a functional whole. Sorry; we thought this was a scientific baseball diamond.
It is. So what is your explanation for the functional information in the simplest life? Your paper admits that “ATP links energy metabolism with genetic information.” What is the source of that genetic information? Uh, some sort of intermediate or other.
The referees convene and shout out, “GAME OVER!”
