 Life Sciences
Life Sciences
 Physics, Earth & Space
Physics, Earth & Space
Uracil Discovered on Asteroid Ryugu — What Does It Mean for the Origin of Life?

Recently, chemical composition data were obtained from samples retrieved by the Japanese spacecraft Hayabusa2 that was landed in two locations on the asteroid (162173) Ryugu. In December 2020 Hayabusa2 successfully returned to Earth with its precious pristine samples, uncontaminated by residues from Earth (except maybe some metallic material originating from the collection device). Published in Science, early analysis of organic compounds extracted from the collected samples included significantly racemic mixtures of several amino acids, indicating that these samples were relatively free of Earthly contamination from biopolymers.1 (All proteins in life are made of racemically pure L-amino acids.) Prior analysis of meteorites could not boast of such purity uncontaminated by Earth’s biological products.
Therefore, these Ryugu samples appear to be our first chance to examine which organic compounds may be produced in a prebiotic setting in our solar system. In addition, we don’t have to rely on uncertain estimates of the conditions on Earth when the solar system was forming. Asteroids containing significant amounts of carbon, never visited by extraterrestrials like us, may provide a reasonable idea of what abiotic chemistry can produce.
It Came from Outer Space?
A recent article in Nature Communications reported that uracil, one of the nucleobases found in RNA, was identified in these pristine Ryugu samples.2 For those who place their bets on the RNA world hypothesis, this is a significant finding, at least in their opinion. They finally have evidence that at least one of the nucleobases of RNA has been discovered outside of Earth, thus, they say, upholding the notion that our biochemistry could have been seeded from outer space! As Live Science summarizes, “After becoming trapped on asteroids like Ryugu, these molecules may have eventually hitched a ride to Earth via meteorite impacts, where they sparked the first stirrings of life in primordial oceans.”
This may be our earliest chance to remark on valid data of prebiotic chemicals free of Earthly contamination. So it would be logical to consider first the initial chemical analysis described in Science to broadly classify all organic compounds, identified using two sensitive analytical methods. Concerning the building blocks of life, they found several amino acids, but all in racemic mixtures. This is precisely what chemists predict. It is extremely difficult to produce optically pure compounds from smaller compounds. The chemistry of mirror-imaged compounds is exactly the same, differing only in the spatial orientation of covalently bonded atoms (analogous to your right and left hands being equivalent). Life only uses one of these chemical forms to make proteins, RNA, DNA, complex carbohydrates and many lipids.
Only the Simplest Amino Acids
On Ryugu just some of the simplest amino acids were detected, including glycine, D/L-alanine, D/L-serine, and D/L-valine, along with other amino acids not used to build proteins (Fig. 1). These results agree well with the earlier experiments by Stanley Miller and others where simple organic structures were readily produced in prebiotic simulations. However, over eight amino acids with more complex critical functional groups have still not resulted among the various permutations of prebiotic reactions tested. It’s not just dealing with racemic mixtures that confounds the supporters of abiogenesis, but how to form those more elaborate amino acids whose side chains play critical roles in the activity and structure of all proteins.
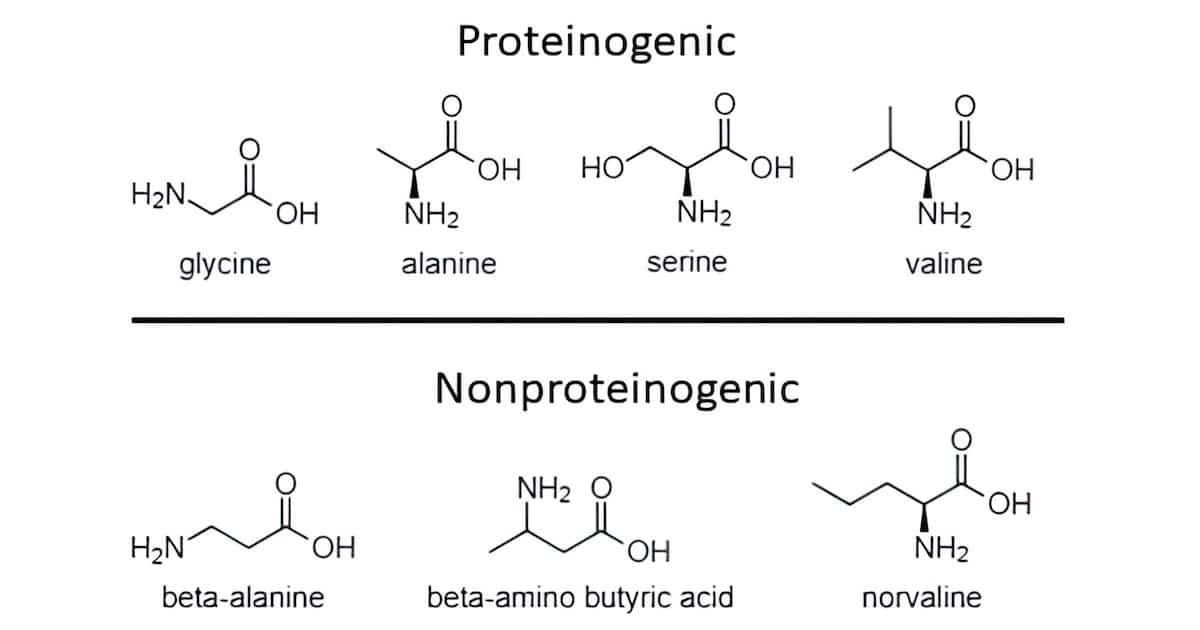
The chemical analysis also reported thousands of organic compounds, classified in multiple groups, that may or may not be found in the context of living organisms. If a primordial soup were to originate from this mixture, any biomolecules would have to contend with a myriad of possible side reactions with a variety of reactive compounds competing for the rights to produce a biopolymer. Would the situation be different if the asteroid material were to simply seed the Earth with these needed building blocks? The competing contaminants still far outnumber the biologically relevant molecules.
The RNA Route
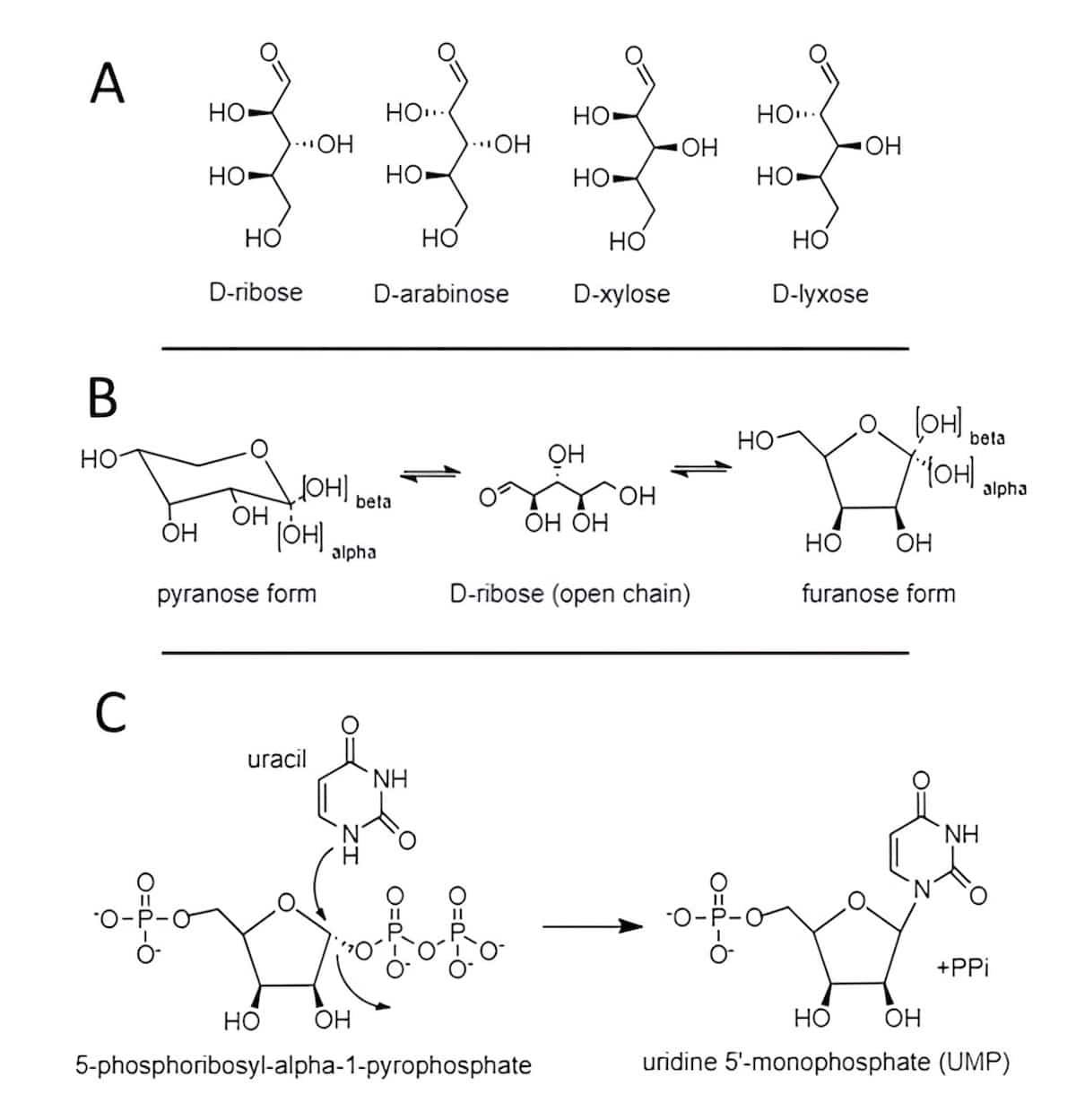
Let’s consider whether the prospects are better if we take the RNA route. Uracil was clearly identified from Ryugu. The engineering threshold to form nucleotides, the building blocks for RNA, is much higher than that for proteins. The core unit to which nucleobases and phosphate are bound is the 5-carbon sugar D-ribose. Several mechanisms have been proposed to explain how carbohydrates may have originated in an abiotic environment.3 The challenges to integrate D-ribose into nucleotides abiotically can be summarized as three major chemical barriers. 1) Abiotic production of five-carbon sugars will yield four chemically equivalent stereoisomers in both the D and L forms, thus resulting in eight stereoisomers at approximately equal levels (Fig. 2A). How does D-ribose get selected through random chemistry without even considering that longer chain sugars will also be present? 2) Ribose can interconvert from an open-chain form to a six-membered ring structure (pyranose form) or to a five-membered ring (furanose), both of which present as alpha and beta configurations at carbon 1 (Fig. 2B). At equilibrium the pyranose form comprises 80 percent while the furanose form is 20 percent of the ribose. RNA uses the furanose form, so how does this minor component win out in any abiotic reactions? 3) Nitrogen at position 1 of uracil needs to be bonded with carbon 1 of D-ribose in a beta configuration through a thermodynamically unfavored reaction. How can this reaction occur abiotically?
Ignoring Conundrums
While the first two conundrums are most often sidestepped by those upholding the RNA-world philosophy, the latter reaction is not an impossible task so we will consider how life manages this feat. Most cells can salvage RNA or DNA building blocks normally obtained nutritionally following digestion. The liberated nucleobases can be coupled using D-ribose charged with a pyrophosphate group at carbon 1 in the alpha configuration. Notice the specificity life uses where neither the beta configuration nor the pyranose ring form will work for this reaction. This substrate permits a specifically oriented approach by the appropriate nitrogen of the nucleobase, directed completely by the respective enzyme, to effect displacement of pyrophosphate. This results in the nucleobase bonding to carbon 1 in the beta configuration (Fig. 2C). The release of pyrophosphate fulfills the thermodynamic requirements of this elaborate reaction.
Attempts have been made to carry out this reaction under purportedly abiotic conditions. These efforts led to some ingenious planning to devise new chemical synthetic schemes involving electrospray of microdroplets containing D-ribose, phosphate, and nucleobases.4 Researchers provide evidence proposing how the microdroplets might make this reaction more thermodynamically favored. It’s feasible for all four nucleotides to be made via this route.
Other Serious Concerns
But this report did not address the other serious concerns already discussed. They used pure D-ribose, not a mixture of sugars as would be expected prebiotically, and minimally not D/L-ribose. The alpha/beta configuration of the products, or their ring structures, were not indicated (most likely resulting in mixtures of all possible products). It thus becomes difficult to evaluate the relevance of this reaction to producing biologically viable RNA building blocks. Finally, the yields of desired products by this mechanism were low, at 2.5 percent or less. While the attempt to produce RNA building blocks via an abiotic mechanism is to be applauded, this still falls far short of what life needs to get started from the complex mixture of organic compounds present in a prebiotic world.
Readers are encouraged to investigate further to more fully understand the difficult issues involved in forming life using undirected organic chemistry alone. Chemist Dr. James Tour at Rice University, for one, has addressed abiogenesis including in discussions on his YouTube channel. See also his chapter in the freely downloadable book Science and Faith in Dialogue.
Notes
- Naraoka H, Takano Y, Dworkin JP, Oba Y, Hamase K, Furusho A, et al. Soluble organic molecules in samples of the carbonaceous asteroid (162173) Ryugu. Science 2023; 379(6634):eabn9033 doi 10.1126/science.abn9033.
- Oba Y, Koga T, Takano Y, Ogawa NO, Ohkouchi N, Sasaki K, et al. Uracil in the carbonaceous asteroid (162173) Ryugu. Nat Commun 2023; 14(1):1292 doi 10.1038/s41467-023-36904-3.
- Yadav M, Kumar R, Krishnamurthy R. Chemistry of Abiotic Nucleotide Synthesis. Chemical Reviews 2020; 120(11):4766-805 doi 10.1021/acs.chemrev.9b00546.
- Nam I, Nam HG, Zare RN. Abiotic synthesis of purine and pyrimidine ribonucleosides in aqueous microdroplets. Proc Natl Acad Sci U S A 2018; 115(1):36-40 doi 10.1073/pnas.1718559115.
