 Intelligent Design
Intelligent Design
Why Proteins Aren’t Easily Recombined
There seems to be an idea floating about among some biologists that it is easy to recombine protein domains or swap bits of protein structure to generate new function. I suppose it comes from looking at simplified drawings of protein structure, and forgetting about the detailed atomic interactions required.
For non-biologists, let me explain why proteins aren’t easily recombined. A protein fold is typically composed of smaller structural elements called alpha helices or beta sheets, with unstructured loops of protein connecting them. These elements adopt a stereotyped pattern of folding because of hydrogen bonding patterns between amino acids. The illustration below from Axe (2010) shows these hydrogen bonding patterns as red dashed lines between the linked amino acids. For clarity, the side chains of each amino acid are faded out, while the carbon backbone trace is in full color.
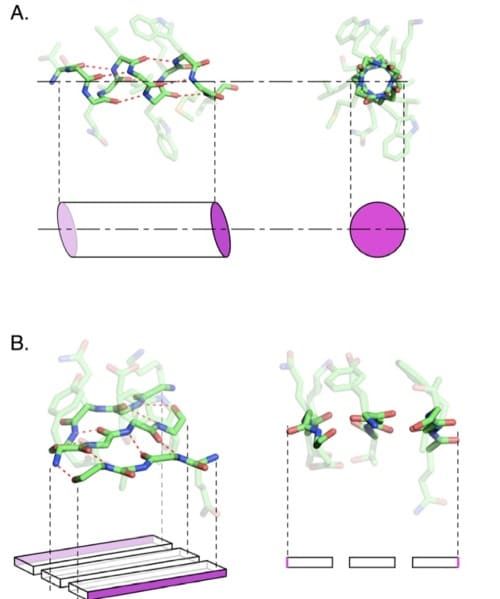
Below each coil (a) or sheet (b) is a simplified geometric shape that illustrates how the element assembles and what edges are available for extension (magenta faces). We see each kind of structure from the side (on the left) and face on (on the right).
It is important to know that different amino acid combinations can form each of these elements — many different sequence combinations can form alpha helices or beta sheets. As a result, each particular helix or sheet has a distinct set of side chains sticking out from it, requiring a distinct set of chemical interactions with any nearby protein sequence. Thus, coils and sheets are sequence-dependent structural elements within protein folds. You can’t swap them around like lego bricks.
This necessarily means that when you bring new secondary structure elements into contact by some sort of rearrangement, they will be unlikely to form a stable three-dimensional fold without significant modification.
But you don’t have to take my word for it — it is possible to test these things. My next post will introduce one such experiment.
Cross-posted at Biologic Perspectives.


