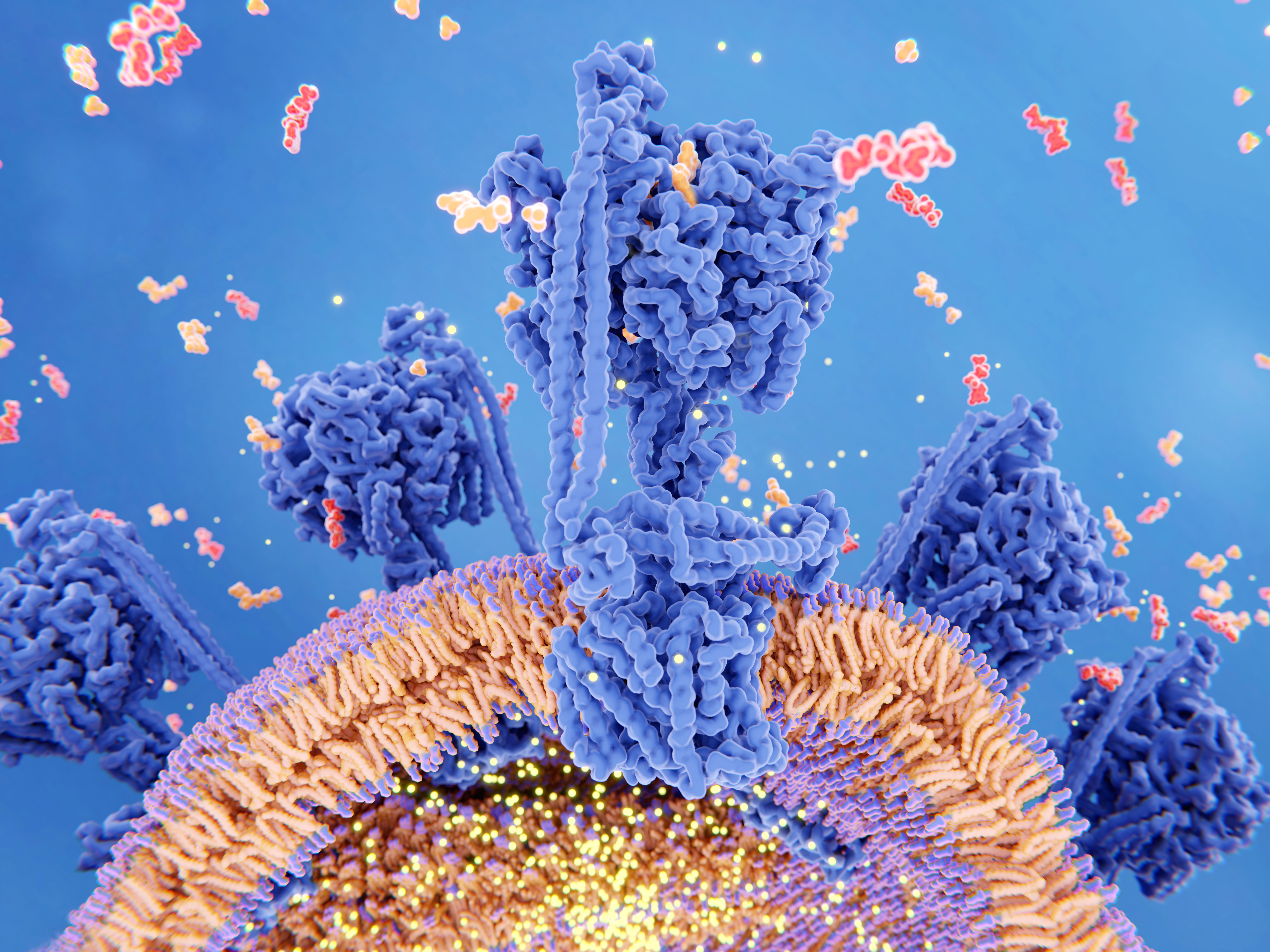
Informational
Molecular Machines
Long before the advent of modern technology, students of biology compared the workings of life to machines.1 In recent decades, this comparison has become stronger than ever. As a paper in Nature Reviews Molecular Cell Biology states, “Today biology is revealing the importance of ‘molecular machines’ and of other highly organized molecular structures that carry out the complex physico-chemical processes on which life is based.”2 Likewise, a paper in Nature Methods observed that “[m]ost cellular functions are executed by protein complexes, acting like molecular machines.”3
What are Molecular Machines?
A molecular machine, according to an article in the journal Accounts of Chemical Research, is “an assemblage of parts that transmit forces, motion, or energy from one to another in a predetermined manner.”4 A 2004 article in Annual Review of Biomedical Engineering asserted that “these machines are generally more efficient than their macroscale counterparts,” further noting that “[c]ountless such machines exist in nature.”5 Indeed, a single research project in 2006 reported the discovery of over 250 new molecular machines in yeast alone!6
Molecular machines have posed a stark challenge to those who seek to understand them in Darwinian terms as the products of an undirected process. In his 1996 book Darwin’s Black Box: The Biochemical Challenge to Evolution, biochemist Michael Behe explained the surprising discovery that life is based upon machines:
Shortly after 1950 science advanced to the point where it could determine the shapes and properties of a few of the molecules that make up living organisms. Slowly, painstakingly, the structures of more and more biological molecules were elucidated, and the way they work inferred from countless experiments. The cumulative results show with piercing clarity that life is based on machines — machines made of molecules! Molecular machines haul cargo from one place in the cell to another along “highways” made of other molecules, while still others act as cables, ropes, and pulleys to hold the cell in shape. Machines turn cellular switches on and off, sometimes killing the cell or causing it to grow. Solar-powered machines capture the energy of photons and store it in chemicals. Electrical machines allow current to flow through nerves. Manufacturing machines build other molecular machines, as well as themselves. Cells swim using machines, copy themselves with machinery, ingest food with machinery. In short, highly sophisticated molecular machines control every cellular process. Thus, the details of life are finely calibrated and the machinery of life enormously complex.7
Behe then posed the question, “Can all of life be fit into Darwin’s theory of evolution?,” and answered: “The complexity of life’s foundation has paralyzed science’s attempt to account for it; molecular machines raise an as-yet impenetrable barrier to Darwinism’s universal reach.”8
Even those who disagree with Behe’s answer to that question have marveled at the complexity of molecular machines. In 1998, former president of the U.S. National Academy of Sciences Bruce Alberts wrote the introductory article to an issue of Cell, one of the world’s top biology journals, celebrating molecular machines. Alberts praised the “speed,” “elegance,” “sophistication,” and “highly organized activity” of “remarkable” and “marvelous” structures inside the cell. He went on to explain what inspired such words:
The entire cell can be viewed as a factory that contains an elaborate network of interlocking assembly lines, each of which is composed of a set of large protein machines. . . . Why do we call the large protein assemblies that underlie cell function protein machines? Precisely because, like machines invented by humans to deal efficiently with the macroscopic world, these protein assemblies contain highly coordinated moving parts.9
Likewise, in 2000 Marco Piccolini wrote in Nature Reviews Molecular Cell Biology that “extraordinary biological machines realize the dream of the seventeenth-century scientists … that ‘machines will be eventually found not only unknown to us but also unimaginable by our mind.’” He notes that modern biological machines “surpass the expectations of the early life scientists.”10
A few years later, a review article in the journal Biological Chemistry demonstrated the difficulty evolutionary scientists have faced when trying to understand molecular machines. Essentially, they must deny their scientific intuitions when trying to grapple with the complexity of the fact that biological structures appear engineered to the schematics of blueprints:
Molecular machines, although it may often seem so, are not made with a blueprint at hand. Yet, biochemists and molecular biologists (and many scientists of other disciplines) are used to thinking as an engineer, more precisely a reverse engineer. But there are no blueprints … ‘Nothing in biology makes sense except in the light of evolution’: we know that Dobzhansky (1973) must be right. But our mind, despite being a product of tinkering itself strangely wants us to think like engineers.11
But do molecular machines make sense in the light of undirected Darwinian evolution? Does it make sense to deny the fact that machines show all signs that they were designed? Michael Behe argues that in fact molecular machines meet the very test that Darwin posed to falsify his theory, and indicate intelligent design.
Darwin knew his theory of gradual evolution by natural selection carried a heavy burden: “If it could be demonstrated that any complex organ existed which could not possibly have been formed by numerous, successive, slight modifications, my theory would absolutely break down.”
… What type of biological system could not be formed by “numerous successive slight modifications”? Well, for starters, a system that is irreducibly complex. By irreducibly complex I mean a single system which is composed of several interacting parts that contribute to the basic function, and where the removal of any one of the parts causes the system to effectively cease functioning.12
Molecular machines are highly complex and in many cases we are just beginning to understand their inner workings. As a result, while we know that many complex molecular machines exist, to date only a few have been studied sufficiently by biologists so that they have directly tested for irreducible complexity through genetic knockout experiments or mutational sensitivity tests. What follows is a non-exhaustive list briefly describing 40 molecular machines identified in the scientific literature. The first section will cover molecular machines that scientists have argued show irreducible complexity. The second section will discuss molecular machines that may be irreducibly complex, but have not been studied in enough detail yet by biochemists to make a conclusive argument.
Selected List of Molecular Machines
I. Molecular Machines that Scientists Have Argued Show Irreducible Complexity
1. Bacterial Flagellum
The flagellum is a rotary motor in bacteria that drives a propeller to spin, much like an outboard motor, powered by ion flow to drive rotary motion. Capable of spinning up to 100,000 rpm,13 one paper in Trends in Microbiology called the flagellum “an exquisitely engineered chemi-osmotic nanomachine; nature’s most powerful rotary motor, harnessing a transmembrane ion-motive force to drive a filamentous propeller.”14 Due to its motor-like structure and internal parts, one molecular biologist wrote in the journal Cell, “[m]ore so than other motors, the flagellum resembles a machine designed by a human.”15 Genetic knockout experiments have shown that the E. coli flagellum is irreducibly complex with respect to its approximately 35 genes.16 Despite the fact that this is one of the best studied molecular machines, a 2006 review article in Nature Reviews Microbiology admitted that “the flagellar research community has scarcely begun to consider how these systems have evolved.”17
2. Eukaryotic Cilium
The cilium is a hair-like, or whip-like structure that is built upon a system of microtubules, typically with nine outer microtubule pairs and two inner microtubules. The microtubules are connected with nexin arms and a paddling-like motion is instigated with dynein motors.18 These machines perform many functions in Eukaryotes, such as allowing sperm to swim or removing foreign particles from the throat. Michael Behe observes that the “paddling” function of the cilium will fail if it is missing any microtubules, connecting arms, or lacks sufficient dynein motors, making it irreducibly complex.19
3. Aminoacyl-tRNA Synthetases (aaRS)
aaRS enzymes are responsible for charging tRNAs with the proper amino acid so they can accurately participate in the process of translation. In this function, aaRSs are an “aminoacylation machine.”20 Most cells require twenty different aaRS enzymes, one for each amino acid, without which the transcription/translation machinery could not function properly.21 As one article in Cell Biology International stated: “The nucleotide sequence is also meaningless without a conceptual translative scheme and physical ‘hardware’ capabilities. Ribosomes, tRNAs, aminoacyl tRNA synthetases, and amino acids are all hardware components of the Shannon message ‘receiver’. But the instructions for this machinery is itself coded in DNA and executed by protein ‘workers’ produced by that machinery. Without the machinery and protein workers, the message cannot be received and understood. And without genetic instruction, the machinery cannot be assembled.”22 Arguably, these components form an irreducibly complex system.23
4. Blood clotting cascade
The blood coagulation system “is a typical example of a molecular machine, where the assembly of substrates, enzymes, protein cofactors and calcium ions on a phospholipid surface markedly accelerates the rate of coagulation.”24 According to a paper in BioEssays, “the molecules interact with cell surface (molecules) and other proteins to assemble reaction complexes that can act as a molecular machine.”25 Michael Behe argues, based upon experimental data, that the blood clotting cascade has an irreducible core with respect to its components after its initiation pathways converge.26
5. Ribosome
The ribosome is an “RNA machine”27 that “involves more than 300 proteins and RNAs”28 to form a complex where messenger RNA is translated into protein, thereby playing a crucial role in protein synthesis in the cell. Craig Venter, a leader in genomics and the Human Genome Project, has called the ribosome “an incredibly beautiful complex entity” which requires a “minimum for the ribosome about 53 proteins and 3 polynucleotides,” leading some evolutionist biologists to fear that it may be irreducibly complex.29
6. Antibodies and the Adaptive Immune System
Antibodies are “the ‘fingers’ of the blind immune system — they allow it to distinguish a foreign invader from the body itself.”30 But the processes that generate antibodies require a suite of molecular machines.31 Lymphocyte cells in the blood produce antibodies by mixing and matching portions of special genes to produce over 100,000,000 varieties of antibodies.32 This “adaptive immune system” allows the body to tag and destroy most invaders. Michael Behe argues that this system is irreducibly complex because many components must be present for it to function: “A large repertoire of antibodies won’t do much good if there is no system to kill invaders. A system to kill invaders won’t do much good if there’s no way to identify them. At each step we are stopped not only by local system problems, but also by requirements of the integrated system.”33
II. Additional Molecular Machines
7. Spliceosome
The spliceosome removes introns from RNA transcripts prior to translation. According to a paper in Cell, “In order to provide both accuracy to the recognition of reactive splice sites in the pre-mRNA and flexibility to the choice of splice sites during alternative splicing, the spliceosome exhibits exceptional compositional and structural dynamics that are exploited during substrate-dependent complex assembly, catalytic activation, and active site remodeling.”34 A 2009 paper in PNAS observed that “[t]he spliceosome is a massive assembly of 5 RNAs and many proteins”35 — another paper suggests “300 distinct proteins and five RNAs, making it among the most complex macromolecular machines known.”36
8. F0F1 ATP Synthase
According to cell biologist and molecular machine modeler David Goodsell, “ATP synthase is one of the wonders of the molecular world.”37 This protein-based molecular machine is actually composed of two distinct rotary motors which are joined by a stator: As the F0 motor is powered by protons, it turns the F1 motor. This kinetic energy is used like a generator to synthesize adenosine triphosphate (ATP), the primary energy carrying molecule of cells.38
9. Bacteriorhdopsin
Bacteriorhodopsin “is a compact molecular machine” that uses that sunlight energy to pump protons across a membrane.39 Embedded in the cell membrane, it consists of seven helical structures that span the membrane. It also contains retinal, a molecule which changes shape after absorbing light. Photons captured by retinal are forced through the seven helices to the outside of the membrane.40 When protons flow back through the membrane, ATP is formed.
10. Myosin
Myosin is a molecular motor that moves along a “track” — in this case actin filaments — to form the basis of muscle movement or to transport cargoes within the cell.41 Muscles use molecular machines like myosin to “convert chemical energy into mechanical energy during muscle contraction.”42 In fact, muscle movement requires the “combined action of trillions of myosin motors.”43
11. Kinesin Motor
Much like myosin, kinesin is a protein machine that binds to and carries cargoes by “crawl[ing] hand-over-hand along a microtubule” in the cell.44 Kinesins are powerful enough to drag large cellular organelles through the cell as well as vesicles or aid in assembly of bipolar spindles, or depolymerization of microtubules.45
12. Tim/Tom Systems
Tim or Tom systems are selective protein pump machines that import proteins across the inner (Tim) and outer (Tom) membranes of mitochondria into the interior matrix of the mitochondria.46
13. Calcium Pump
The calcium pump is an “amazing machine with several moving parts“ that transfers calcium ions across the cell membrane. It is a machine that uses a 4-step cycle during the pump process.47
14. Cytochrome C Oxidase
Cytochrome C Oxidase qualifies as a molecular machine “since part of the redox free energy is transduced into a proton electrochemical gradient.”48 The enzyme’s function is to carefully control the final steps of food oxidation by combining electrons with oxygen and hydrogen to form water, thereby releasing energy. It uses copper and iron atoms to aid in this process.49
15. Proteosome
The proteosome is a large molecular machine whose parts must be must be carefully assembled in a particular order. For example, the 26S proteosome has 33 distinct subunits which enable it to perform its function to degrade and destroy proteins that have been misfolded in the cell or otherwise tagged for destruction.50 One paper suggested that a particular eukaryotic proteasome “is the core complex of an energy-dependent protein degradation machinery that equals the protein synthesis machinery in its complexity.”51
16. Cohesin
Cohesin is molecular machine “multisubunit protein complex”52 and “a macromolecular complex that links sister chromatids together at the metaphase plate during mitosis.”53
17. Condensin
Condensin is a molecular machine that helps to condense and package chromosomes for cell replication. It is a five subunit complex, and is “the key molecular machine of chromosome condensation.”54
18. ClpX
ClpX is a molecular machine that uses ATP to both unfold proteins and then transport unfolded proteins into another complex in the cell. It moves these proteins into the ClpP complex.55
19. Immunological Synapse
The immunological synapse is a molecular machine that serves as an interface to activate of T cells. Once an immunological synapse is completely formed, T Cells are activated and proliferate, sparking key part of the immune response.56
20. Glideosome
The glideosome is a “macromolecular complex” and an “elaborate machine”57 whose function is to allow protozoa to rely on gliding motility over various substrates.
21. Kex2
Kex2 is a molecular machine that facilitates cell fusion during the mating of yeast; it likely works by degrading cell walls.58
22. Hsp70
Hsp70 is one of many molecular machines that serve as chaperones that not only assist other proteins in reaching a proper functional conformation (i.e. proper folding) but also helping them to be transported to the proper location in the cell.59
23. Hsp60
Hsp60 is another chaperone machine – it is tailored to provide “an enclosed environment for folding proteins which totally protects them as they fold.”60 It is composed of multiple proteins which form a barrel shaped structure with a cap.61 Once an unfolded protein is inside, it can fold properly.
24. Protein Kinase C
Protein Kinase C is a molecular machine that is activated by certain calcium and diacylglycerol signals in the cell. It thus acts as an interpreter of electrical signals, as one paper in Cell wrote: “This decoding mechanism may explain how cPKC isoforms can selectively control different cellular processes by relying on selective patterns of calcium and diacylglycerol signals.”62
25. SecYEG PreProtein Translocation Channel
The SecYE complex is vital to the operation of “translocation machinery” which works to move molecules across membranes in the cell.63
26. Hemoglobin
Molecular machine modeller David Goodsell observes that “Hemoglobin is a remarkable molecular machine that uses motion and small structural changes to regulate its action.”64 Hemoglobin uses iron within its protein structure to carry oxygen from the lungs to the rest of the body through the blood.
27. T4 DNA Packaging Motor
The T4 DNA is one of various packaging motors that are “powerful molecular motors” which emplace viral genomes into capsules called procapsids.65 Once viral genome packaging is complete, “the DNA packaging motor is released and the separately assembled tail is attached to produce the mature infectious viral particle.”66
28. Smc5/Smc6
Smc5/Smc6 is a complex machine that is involved with the structural maintenance of chromosomes with regards to cohesions and condensins,67 and works to remove cohesin from damaged chromosomes prior to chromosomal separation,68 and may also work to repair and untangle DNA.69
29. Cytplasmic Dynein
Cytplasmic dynein is a machine involved with cargo transport and movement cell that functions like a motor with a “power stroke.”70 In particular, it transports nuclei in fungi and neurons in mammalian brains.71
30. Mitotic Spindle Machine
The mitotic spindle is a highly dynamic self-assembling complex molecular machine composed of tubulin, motors, and other molecules which assembles around the chromosomes and segregates them into daughter cells during mitosis.72
31. DNA Polymerase
The DNA polymerase is a multiprotein machine that creates a complementary strand of DNA from a template strand.73 The DNA polymerase is not only the “central component of the DNA replication machinery,”74 but it “plays the central role in the processes of life,”75 since it is responsible for the copying of DNA from generation to generation. During the polymerization process, it remains tethered to the DNA using a protein-based sliding clamp.76 It is extremely accurate, making less than one mistake per billion bases, aided by its ability to proofread and fix mistakes.77
32. RNA Polymerase
Like its DNA polymerase counterpart, the function of the RNA polymerase is to create a messenger RNA strand from a DNA template strand. Called “a huge factory with many moving parts,”78 it is a “directional machine and, indeed, as a molecular motor” where it functions “as a dynamic, fluctuating, molecular motor capable of producing force and torque.”79
33. Kinetochore
The kinetochore is a “proteinaceous structure that assembles on centromeric chromatin and connects the centromere to spindle microtubules.”80 Called a “macromolecular protein machine,”81 it is composed of over 80 protein components;82 it aids in separating chromosomes during cell division.
34. MRX Complex
The MRX complex forms telomere length counting machinery that measures the integrity of telomeres, the structures that protect the ends of eukaryotic chromosomes. Properly measuring telomere length is vital to ensure proper cell lifetime and genome stability.83 Yeast use the MRX complex via a “’protein-counting’ mechanism whereby higher numbers of proteins bound by a longer telomere repeat tract ultimately inhibit telomerase activity at that particular telomere.”84
35. Apoptosome / Caspase
While many molecular machines keep a cell alive, there are even machines that are programmed to cause cell death, or apoptosis. Cell death must be carefully timed so that cells die when they need to be replaced. According to David Goodsell, “Caspases are the executioners of apoptosis,” and they work by destroying specific proteins in the right order so as to “disassemble the cell in an orderly manner.”85 Caspases can be part of a “death machine” called the apoptosome,86 a molecular machine which receives signals indicating cellular stress and then initiates cell death, including activity of caspases.
36. Type III Secretory System
This machine, often called the T3SS, is a toxin injection machine used by predatory bacteria to deliver deadly toxins into other cells.87 It is composed of subunits that are machines, such as the injectisome nanomachine.88
37. Type II Secretion Apparatus
The T2SS is a complex nanomachine that translocates proteins across the outer membrane of a bacterium.89
38. Helicase/Topoisomerase Machine
The helicase and topoisomerase machines work together to properly unwrap or unzip DNA prior to transcription of DNA into mRNA or DNA replication.90 Topoisomerase performs this function by cutting one DNA strand and then holding on to the other while the cut strand unwinds.91
39. RNA degradasome
The RNA degradasome “multiprotein complex involved in the degradation of mRNA”92 or trimming RNAs into their active forms93 in E. coli bacteria. Its large size “would readily qualify [it] as a supramolecular machine dedicated to RNA processing and turnover.”94
40. Photosynthetic system
The processes that plants use to convert light into chemical energy a type of molecular machines.95 For example, photosystem 1 contains over three dozen proteins and many chlorophyll and other molecules which convert light energy into useful energy in the cell. “Antenna” molecules help increase the amount of light aborbed.96 Many complex molecules are necessary for this pathway to function properly.
References Cited
- See Marco Piccolino, “Biological machines: from mills to molecules,” Nature Reviews Molecular Cell Biology, Vol. 1:149-153 (November, 2000).
- Marco Piccolino, “Biological machines: from mills to molecules,” Nature Reviews Molecular Cell Biology, Vol. 1:149-153 (November, 2000).
- Thomas Köcher & Giulio Superti-Furga, “Mass spectrometry-based functional proteomics: from molecular machines to protein networks,” Nature Methods, Vol. 4(10):807-815 (October, 2007).
- Tinh-Alfredo V. Khuong, Jose E. Junez, Carlos E. Godinez, and Miguel A. Garcia-Garibay, “Crystalline Molecular Machines: A Quest Toward Solid-State Dynamics and Function,” Accounts of Chemical Research, Vol. 39(6):413-422 (2006).
- C. Mavroidis, A. Dubey, and M.L. Yarmush, “Molecular Machines,” Annual Review of Biomedical Engineering, Vol. 6:363-395 (2004).
- See “The Closest Look Ever At The Cell’s Machines,” ScienceDaily.com (January 24, 2006).
- Michael Behe, Darwin’s Black Box: The Biochemical Challenge to Evolution, pp. 4-5 (Free Press, 1996).
- Michael Behe, Darwin’s Black Box: The Biochemical Challenge to Evolution, p. 5 (Free Press, 1996).
- Bruce Alberts, “The Cell as a Collection of Protein Machines: Preparing the Next Generation of Molecular Biologists,” Cell, Vol. 92:291 (February 6, 1998).
- Marco Piccolino, “Biological machines: from mills to molecules,” Nature Reviews Molecular Cell Biology, Vol. 1:149-153 (November, 2000).
- Walter Neupert, “Highlight: Molecular Machines,” Biological Chemistry, Vol. 386(8):711(August, 2005).
- Michael Behe, Darwin’s Black Box: The Biochemical Challenge to Evolution, p. 39 (Free Press, 1996).
- Seiji Kojima and David F. Blair, “The Bacterial Flagellar Motor: Structure and Function of a Complex Molecular Machine,” International Review of Cytology, Vol. 233:93-134 (2004).
- Mark J. Pallen, Charles W. Penn and Roy R. Chaudhuri, “Bacterial flagellar diversity in the post-genomic era,” Trends in Microbiology, Vol. 13(4):143-149 (April, 2005).
- David J. DeRosier, “The turn of the screw: The bacterial flagellar motor,” Cell, Vol. 93: 17-20 (1998).
- See Transcript of Kitzmiller v. Dover Trial, Afternoon Session (Nov. 3, 2005), pp. 99-108.
- Mark J. Pallen and Nicholas J. Matzke, “From The Origin of Species to the Origin of Bacterial Flagella,” Nature Reviews Microbiology, Vol. 4 (September 5, 2006).
- Daniela Nicastro, J. Richard McIntosh, and Wolfgang Baumeister, “3D structure of eukaryotic flagella in a quiescent state revealed by cryo-electron tomography,” Proceedings of the U.S. National Academy of Sciences, Vol. 102:15889-15894 (November 1, 2005).
- Michael Behe, Darwin’s Black Box: The Biochemical Challenge to Evolution, p. 65 (Free Press, 1996).
- M.T. Norcum, C. L. Wolfe, and J.A. Warrington, “Three-dimensional Working Model of the Multienzyme Aminoacyl-tRNA Synthetase Complex Determined by Computational Microscopy,” Microsc Microanal, Vol. 11:164-165 (2005).
- See David Goodsell, “Aminoacyl-tRNA Synthetases,” Molecule of the Month at Protein Data Bank (April, 2001).
- J.T. Trevors and D.L. Abel, “Chance and necessity do not explain the origin of life,” Cell Biology International, Vol. 28: 729-739 (2004).
- See Stephen C. Meyer, Signature in the Cell: DNA and the Evidence for Intelligent Design, p. 246-249 (HarperOne, 2009).
- Henri M.H. Spronk, José W.P. Govers-Riemslag, and Hugo ten Cate, “The blood coagulation system as a molecular machine,” BioEssays, Vol. 25:1220-1228 (2003).
- Henri M.H. Spronk, José W.P. Govers-Riemslag, and Hugo ten Cate, “The blood coagulation system as a molecular machine,” BioEssays, Vol. 25:1220-1228 (2003).
- Michael Behe, Darwin’s Black Box: The Biochemical Challenge to Evolution, p 86 (Free Press, 1996).
- Thomas R. Cech, “Crawling Out of the RNA World,” Cell, Vol. 136:599-602 (February 20, 2009).
- Jonathan P Staley and John L Woolford, Jr, “Assembly of ribosomes and spliceosomes: complex ribonucleoprotein Machines,” Current Opinion in Cell Biology, Vol. 21(1):109-118 (February, 2009).
- “Life: What A Concept!” (The Edge Foundation, 2008).
- Michael Behe, Darwin’s Black Box: The Biochemical Challenge to Evolution, p. 120 (Free Press, 1996).
- Marco Piccolino, “Biological machines: from mills to molecules,” Nature Reviews Molecular Cell Biology, Vol. 1:149-153 (November, 2000).
- See David Goodsell, “Antibodies,” Molecule of the Month at Protein Data Bank (September, 2001).
- Michael Behe, Darwin’s Black Box: The Biochemical Challenge to Evolution, p. 138 (Free Press, 1996).
- Markus C. Wahl, Cindy L. Will, and Reinhard Lührmann, “The Spliceosome: Design Principles of a Dynamic RNP Machine,” Cell, Vol. 136: 701-718 (February 20, 2009).
- Samuel E. Butcher, “The spliceosome as ribozyme hypothesis takes a second step,” Proceedings of the U.S. National Academy of Sciences, Vol. 106(30):12211-12212 (July 28, 2009).
- Timothy W. Nilsen, “The spliceosome: the most complex macromolecular machine in the cell?,” BioEssays, Vol. 25:1147-1149 (2003).
- See David Goodsell, “The ATP Synthase,” Molecule of the Month at Protein Data Bank (December, 2005).
- C. Mavroidis, A. Dubey, and M.L. Yarmush, “Molecular Machines,” Annual Review of Biomedical Engineering, Vol. 6:363-395 (2004); Paul D. Boyer, “The ATP Synthase—A Splendid Molecular Machine,” Vol. 66:717-749 (1997); Steven M. Block, “Real engines of creation,” Nature, Vol. 386:217-219 (March 20, 1997).
- See David Goodsell, “Bacteriorhodopsin,” Molecule of the Month at Protein Data Bank (March, 2002).
- Werner Kühlbrandt, “Bacteriorhodopsin — the movie,” Nature, Vol. 406:569-570 (August 10, 2009).
- C. Mavroidis, A. Dubey, and M.L. Yarmush, “Molecular Machines,” Annual Review of Biomedical Engineering, Vol. 6:363-395 (2004); Ronald D. Vale, “The Molecular Motor Toolbox for Intracellular Transport,” Cell, Vol. 112:467-480 (February 21, 2003).
- Marco Piccolino, “Biological machines: from mills to molecules,” Nature Reviews Molecular Cell Biology, Vol. 1:149-153 (November, 2000).
- See David Goodsell, “The Calcium Pump,” Molecule of the Month at Protein Data Bank (March, 2004).
- C. Mavroidis, A. Dubey, and M.L. Yarmush, “Molecular Machines,” Annual Review of Biomedical Engineering, Vol. 6:363-395 (2004); Ronald D. Vale, “The Molecular Motor Toolbox for Intracellular Transport,” Cell, Vol. 112:467-480 (February 21, 2003); David Goodsell, “Kinesin,” Molecule of the Month at Protein Data Bank (April, 2005).
- Sharyn A. Endow, “Kinesin motors as molecular machines,” BioEssays, Vol. 25:1212-1219 (2003).
- Nikolaus Pfanner and Michiel Meijer, “Mitochondrial biogenesis: The Tom and Tim machine,” Current Biology, Vol. 7:R100-R103 (1997).
- See David Goodsell, “The Calcium Pump,” Molecule of the Month at Protein Data Bank (March, 2004).
- Francesco Malatesta, Giovanni Antonini, Paolo Sarti, Maurizio Brunori, “Structure and function of a molecular machine: cytochrome c oxidase,” Biophysical Chemistry, Vol. 54: 1-33 (1995).
- See David Goodsell, “Cytochrome c Oxidase,” Molecule of the Month at Protein Data Bank (May, 2000).
- Henrike C. Besche, Andreas Peth, and Alfred L. Goldberg, “Getting to First Base in Proteasome Assembly,” Cell, Vol. 138:25-28 (July 10, 2009).
- Wolfgang Baumeister, Jochen Walz, Frank Zu¨hl, and Erika Seemu¨ller, “The Proteasome: Paradigm of a Self-Compartmentalizing Protease,” Cell, Vol. 92:367-380 (February 6, 1998).
- Jan-Michael Peters, Antonio Tedeschi, and Julia Schmitz, “The cohesin complex and its roles in chromosome biology,” Genes & Development, Vol. 22:3089-3114 (2008).
- Susan Jones and John Sgouros, “The cohesin complex: sequence homologies, interaction networks and shared motifs,” Genome Biology, Vol 2(3) (March 6, 2001); John Mc Intyre, Eric GD Muller, Stefan Weitzer, Brian E Snydsman, Trisha N Davis, and Frank Uhlmann, “In vivo analysis of cohesin architecture using FRET in the budding yeast Saccharomyces cerevisiae,” The EMBO Journal, Vol. 26, 3783-3793 (2007).
- Alexander V. Strunnikov, “Condensin and biological role of chromosome condensation,” Progress in Cell Cycle Research, Vol. 5:361-367, (2003).
- Steven E. Glynn, Andreas Martin, Andrew R. Nager, Tania A. Baker, and Robert T. Sauer, “Structures of Asymmetric ClpX Hexamers Reveal Nucleotide-Dependent Motions in a AAA+ Protein-Unfolding Machine,” Cell, Vol. 139:744-756 (November 13, 2009).
- Arash Grakoui, Shannon K. Bromley, Cenk Sumen, Mark M. Davis, Andrey S. Shaw, Paul M. Allen, Michael L. Dustin, “The Immunological Synapse: A Molecular Machine Controlling T Cell Activation,” Science, Vol. 285:221-227 (July 9, 1999).
- Anthony Keeley and Dominique Soldati, ”The glideosome: a molecular machine powering motility and host-cell invasion by Apicomplexa,” Trends in Cell Biology, Vol.14(10): 528-532 (October, 2004).
- Maxwell G. Heiman, Alex Engel, and Peter Walter, “The Golgi-resident protease Kex2 acts in conjunction with Prm1 to facilitate cell fusion during yeast mating,” The Journal of Cell Biology, Vol. 176:209-222 (January 15, 2007).
- Bernd Bukau and Arthur L. Horwich, “The Hsp70 and Hsp60 Chaperone Machines,” Cell, Vol. 92: 351-366 (February 6, 1998).
- See David Goodsell, “Chaperones,” Molecule of the Month at Protein Data Bank (August, 2002).
- “The Birth, Assembly, and Death of Proteins,” Molecular Biology of the Cell at NCBI.
- Elena Oancea and Tobias Meyer, “Protein Kinase C as a Molecular Machine for Decoding Calcium and Diacylglycerol Signals,” Cell, Vol. 95:307–318 (October 30, 1998).
- Pascal Bessonneau, Veronique Besson, Ian Collinson, and Franck Duong, “The SecYEG preprotein translocation channel is a conformationally dynamic and dimeric structure,” The EMBO Journal, Vol. 21(5): 995-1003 (2002).
- See David Goodsell, “Hemoglobin,” Molecule of the Month at Protein Data Bank (May, 2003).
- Siyang Sun, Kiran Kondabagil, Bonnie Draper, Tanfis I. Alam, Valorie D. Bowman, Zhihong Zhang, Shylaja Hegde, Andrei Fokine, Michael G. Rossmann, and Venigalla B. Rao, “The Structure of the Phage T4 DNA Packaging Motor Suggests a Mechanism Dependent on Electrostatic Forces,” Cell, Vol. 135:1251-1262 (December 26, 2008).
- Siyang Sun, Kiran Kondabagil, Bonnie Draper, Tanfis I. Alam, Valorie D. Bowman, Zhihong Zhang, Shylaja Hegde, Andrei Fokine, Michael G. Rossmann, and Venigalla B. Rao, “The Structure of the Phage T4 DNA Packaging Motor Suggests a Mechanism Dependent on Electrostatic Forces,” Cell, Vol. 135:1251-1262 (December 26, 2008).
- Jordi Torres-Rosell, Ivana Sunjevaric, Giacomo De Piccoli, Meik Sacher, Nadine Eckert-Boulet, Robert Reid, Stefan Jentsch, Rodney Rothstein, Luis Aragón, and Michael Lisby, “The Smc5–Smc6 complex and SUMO modification of Rad52 regulates recombinational repair at the ribosomal gene locus,” Nature Cell Biology, Vol. 9(8):923-931 (August, 2007).
- Emily A. Outwin, Anja Irmisch, Johanne M. Murray, and Matthew J. O’Connell, “Smc5-Smc6-Dependent Removal of Cohesin from Mitotic Chromosomes,” Molecular and Cell Biology, Vol. 29 (16): 4363–4375 (August 2009).
- “A Protein Complex That Untangles DNA,” ScienceDaily.com (July 16, 2006).
- C. Mavroidis, A. Dubey, and M.L. Yarmush, “Molecular Machines,” Annual Review of Biomedical Engineering, Vol. 6:363-395 (2004).
- Ronald D. Vale, “The Molecular Motor Toolbox for Intracellular Transport,” Cell, Vol. 112:467-480 (February 21, 2003).
- E. Karsenti and I. Vernos, “The Mitotic Spindle: A Self-Made Machine,” Science, Vol. 294:543-547 (October 19, 2001); Raja Paul, Roy Wollman, William T. Silkworth, Isaac K. Nardi, Daniela Cimini, and Alex Mogilner, “Computer simulations predict that chromosome movements and rotations accelerate mitotic spindle assembly without compromising accuracy,” Proceedings of the U.S. National Academy of Sciences, Vol. 106(37):15708-15713 (September 15, 2009).
- Jennifer Turner, Manju M. Hingorani, Zvi Kelman, and Mike O’Donnell, “The internal workings of a DNA polymerase clamp-loading machine,” The EMBO Journal, Vol.18:771-783 (1999); “DNA Polymerase: an Active Machine,” The Journal of Biological Chemistry, Vol. 282:e99940 (September 28, 2007).
- Paul J. Rothwell and Gabriel Waksman, “A Pre-equilibrium before Nucleotide Binding Limits Fingers Subdomain Closure by Klentaq1,” The Journal of Biological Chemistry, Vol. 282(39):28884-28892 (September 28, 2007).
- See David Goodsell, “DNA Polymerase,” Molecule of the Month at Protein Data Bank (March, 2000).
- Jennifer Turner, Manju M. Hingorani, Zvi Kelman, and Mike O’Donnell, “The internal workings of a DNA polymerase clamp-loading machine,” The EMBO Journal, Vol.18:771-783 (1999).
- See David Goodsell, “DNA Polymerase,” Molecule of the Month at Protein Data Bank (March, 2000).
- See David Goodsell, “RNA Polymerase,” Molecule of the Month at Protein Data Bank (April, 2003).
- Henri Buc and Terence Strick, RNA polymerases as molecular motors , p. 304 (Royal Society of Chemistry, 2009).
- Steven Henikoff, Kami Ahmad, Harmit S. Malik, “The Centromere Paradox: Stable Inheritance with Rapidly Evolving DNA,” Science, Vol. 293:1098-1102 (August 10, 2001).
- Ajit Joglekar, Kerry Bloom, and E. D. Salmon, “In vivo protein architecture of the eukaryotic kinetochore with nanometer scale accuracy,” Current Biology, Vol. 19(8):694-699 (April 28, 2009).
- Iain M. Cheeseman & Arshad Desai, “Molecular architecture of the kinetochore-microtubule interface,” Nature Reviews Molecular Cell Biology, Vol. 9:33-46 (2008).
- Neal F. Lue, “Closing the Feedback Loop: How Cells ‘Count’ Telomere-Bound Proteins,” Molecular Cell, Vol. 33:413-414 (February 27, 2009).
- Neal F. Lue, “Closing the Feedback Loop: How Cells ‘Count’ Telomere-Bound Proteins,” Molecular Cell, Vol. 33:413-414 (February 27, 2009).
- See David Goodsell, “Caspases,” Molecule of the Month at Protein Data Bank (August, 2004).
- Guy S. Salvesen and Martin Renatus, “Apoptosome: The Seven-Spoked Death Machine,” Developmental Cell, Vol. 2(3): 256-257 (March 1, 2002).
- Jorge E. Galán and Alan Collmer, “Type III Secretion Machines: Bacterial Devices for Protein Delivery into Host Cells,” Science, Vol. 284:1322-1328 (May 21, 1999).
- Guy R. Cornelis, “The type III secretion injectisome,” Nature Reviews Microbiology, Vol. 4:811-825 (November, 2006).
- Guy R. Cornelis, “The type III secretion injectisome,” Nature Reviews Microbiology, Vol. 4:811-825 (November, 2006).
- Michel Duguet, “When helicase and topoisomerase meet!,” Journal of Cell Science, Vol. 110:1345-1350 (1997).
- See David Goodsell, “Topoisomerases,” Molecule of the Month at Protein Data Bank (January, 2006).
- Agamemnon J. Carpousis, “The RNA Degradosome of Escherichia coli: An mRNA-Degrading Machine Assembled on RNase E,” Annual Review of Microbiology, Vol. 61:71-87 (October 2007).
- Maria Jose Marcaida, Mark A. DePristo, Vidya Chandran, Agamemnon J. Carpousis and Ben F. Luisi, “The RNA degradosome: life in the fast lane of adaptive molecular evolution,” Trends in Biochemical Sciences, Vol. 31(7): 359-3365 (July 2006).
- Maria Jose Marcaida, Mark A. DePristo, Vidya Chandran, Agamemnon J. Carpousis and Ben F. Luisi, “The RNA degradosome: life in the fast lane of adaptive molecular evolution,” Trends in Biochemical Sciences, Vol. 31(7): 359-3365 (July 2006).
- Marco Piccolino, “Biological machines: from mills to molecules,” Nature Reviews Molecular Cell Biology, Vol. 1:149-153 (November, 2000).
- See David Goodsell, “Photosystem I,” Molecule of the Month at Protein Data Bank (October, 2001).
