 Evolution
Evolution
 Paleontology
Paleontology
Fossil Friday: The Abrupt Origin of Insectivore Mammals

In December 2022 I began a series of articles at Evolution News about the abrupt origins of placental mammal orders, of which the latest installation was published in February 2023 on lagomorphs and rodents (Bechly 2023c). I promised to continue this series soon and this article shows why it took me so much longer than expected: it literally required the study of hundreds of technical papers to tackle the most obscure and confused grouping in mammal classification. So, this Fossil Friday we finally indeed continue our series and move on to the fourth and final supergroup (cohort), which is called Laurasiatheria. We begin with the order Eulipotyphla that includes insectivores like moles (Talpidae), shrews (Soricidae), and hedgehogs (Erinaceidae), as well as the strange solenodons (Solenodontidae) from Hispaniola and Cuba and the recently extinct West Indian shrews (Nesophontidae) (Orihuela León 2023) and also several extinct groups from the Paleogene. The latter for example include the amphilemurids like the featured Pholidocercus hassiacus from the Eocene oil shale of the famous Messel pit in Germany, which is completely and perfectly preserved in a way that is extremely rare for mammalian fossils which are often represented only by isolated teeth and small skull fragments.
Eulipotyphla is a very diverse order with more than 500 living species that make up about 8-10 percent of mammalian biodiversity (Holmberg 2022). In their biodiversity, they are surpassed only by rodents and bats (Gunnell & Bloch 2008). They are often considered to include some of the most ancient and most primitive placental mammals (e.g., Carroll 1988), even though the order Eulipotyphla is historically the youngest of the mammalian orders and was first named by Waddell et al. (1999).
The Hodgepodge of Insectivore Systematics
The higher classification of insectivore mammals has been a genuine hodgepodge historically, even though all the alternative classifications were based on Darwinian assumptions and extensive comparative studies. Eberle et al. (2014) commented that “the phylogeny of the Insectivora is contentious, with considerable disagreement between morphological and molecular phylogenies, and even among molecular phylogenies.” Unfortunately, we have to sort out this mess, because we first have to know precisely about which kind of group of organisms we are talking, before we can evaluate their fossil record and the question of their abrupt origins. Therefore, here is a recap of this very checkered history (also see Butler 1972, Symonds 2005, Lopatin 2006, Senekowitsch 2013, and the German Wikipedia page Insektenfresser):
A Chronological History of Insectivore Systematics
- Carl von Linné included in his Systema Naturæ (Linnæi 1758), which still represents the foundation of modern biological classification, the hedgehogs, shrews, and moles with pigs, armadillos, and opossums in his order “Bestiae,” based on a shared long nose.
- George Cuvier (1817) included hedgehogs, shrews, and moles with tenrecs and golden moles, as well as some carnivores in his “plantigrades”, but later excluded the carnivores.
- Even though the taxon Insectivora is often falsely credited to Illiger (1811), who used the name Falculata (claw foots) for these animals, it was indeed first established by Bowdich (1821), after Cuvier (1817) had proposed such a grouping under the French vernacular term “les insectivores”.
- Wagner (1855) included hedgehogs, shrews, moles, solenodons, golden moles, tenrecs, flying lemurs, tree shrews, and elephant shrews in Insectivora.
- Based on the presence or absence of a caecum, Peters (1864) instead divided insectivores into two groups that were formally named as suborders Menotyphla (flying lemurs, tree shrews, and elephant shrews) and Lipotyphla (hedgehogs, shrews, moles, solenodons, golden moles, tenrecs) by Ernst Haeckel (1866).
- Based on dental morphology, Gill (1875, 1884-1885) divided extant insectivores into Zalambdodonta (tenrecs, golden moles, otter shrews, and solenodons) and Dilambdodonta (Erinaceoidea, Soricoidea, and Tupaioidea).
- Leche (1885) suggested the removal of Macroscelidea from Menotyphla. Subsequently, all the menotyphlan orders were successively removed from Insectivora and recognized as distinct orders Dermoptera, Scandentia, and Macroscelidea (Butler 1956, 1972).
- Mainly based on views of Thomas Huxley (1880) and other contemporary scientists, Matthew (1909) already suggested that insectivores were the central stock from which other placental mammals descended (see Wyss 1987).
- Gregory (1910) removed the Menotyphla as separate order and elevated Haeckel’s Lipotyphla to subordinal rank within the order Insectivora. He introduced Erinaceomorpha and Soricomorpha as infraorders.
- “Osborn (1910), developing the system proposed by Matthew (1909) and Gregory (1910), divided the order Insectivora into the suborders Lipotyphla, Menotyphla, Hyopsodonta (Hyopsodontidae), and Proglires (Apatemyidae and Mixodectidae)” (Lopatin 2006).
- Jaekel (1911) retained the suborders Menotyphla and Lipotyphla in his order Insectivori. He also included in Lipotyphla the extinct Pantolestidae (today considered as Cimolesta outside Placentalia) and Necrolestidae (today considered as non-therian Meridiolestida).
- Simpson (1931a) retained Tupaioidea (= Menotyphla) in Insectivora, and considered the relationship with Macroscelidae as an open question.
- Based on their different brain structure, Le Gros Clark (1932) abandoned the distinction of Lipotyphla and Menotyphla, but placed elephant shrews and tree shews (Tupaioidea or Menotyphla) in separate superfamilies. He considered tree shrews as close relatives of primates.
- Roux (1947) reviewed the history of the classification of insectivores and only retained tenrecs, golden moles, solenodons, shrews, hedgehogs, and moles as “typical Insectivores”. The monophyly of these remaining Lipotyphla was later supported by various morphological characters (Butler 1956, 1972, MacPhee & Novacek 1993).
- Simpson (1945) called Insectivora the “scrap basket for small mammals” and included 10 families in Lipotyphla: i.e., Leptictidae, Palaeoryctidae, Pantolestidae, Mixodectidae, Deltatheridiidae, Solenodontidae, Erinaceidae, Nyctitheriidae, Soricidae, and Talpidae. He also kept Macroscelidea in Insectivora, but treated Dermoptera as distinct order and included Scandentia in the primate group Lemuriformes and included Proteutheria.
- Saban (1954) elevated the infraorders Erinaceomorpha and Soricomorpha to subordinal rank.
- Butler (1956, 1972, 1988) advocated the name Lipotyphla over Insectivora and suggested a fundamental split of Lipotyphla into a primitive suborder Erinaceomorpha and a more specialized suborder Soricomorpha. Butler (1956: fig. 8) implicitly considered Insectivora as paraphyletic or diphyletic because in his tree he placed Menotyphla closer to Dermoptera and Primates, corresponding to the Archonta concept of Gregory (1910).
- Romer (1966) removed some of the more problematic fossil groups into a new suborder Proteutheria, which was accepted by several subsequent works (e.g., Rose 1981), even though it only obfuscated and protracted the general confusion about the relationships of these groups.
- Van Valen (1967) retained Insectivora and rejected the taxa Lipotyphla, Erinaceomorpha and Soricomorpha. He included the living tree shrews in Proteutheria, and included Proteutheria, Macroscelidea, Dermoptera, and Erinaceota as suborders of Insectivora. In Erinaceota he included the living hedgehogs, moles, and shrews and their fossil relatives. He united tenrecs, golden moles, and solenodons as well as some fossil taxa in a suborder Zalambdodonta of the order Deltatheridia, thus not grouped with the other insectivores.
- Colbert (1969) tentatively listed four suborders but emphasized that this classification is not satisfactory: Proteutheria or Menotyphla (including leptictids, pantolestids, and zalambdalestids), Macroscelidea, Dilambdodonta or Lipotyphla, and Zalambdodonta (tenrecs and golden moles).
- McKenna (1969) said that “early leptictids gave rise to tupaiids, microsyopids, primates, precursors of rodents, pantolestids, etc. An advanced erinaceid-like group fragmented in the late Paleocene to produce a talpid-like group (nyctitheriids, plesiosoricids, talpids, micropternodontids) and again in the late Eocene to produce the soricids.” In terms of modern cladistic classification, this would not just be considered as utterly incorrect but also as meaningless gibberish. But it was based on comparative morphology and Darwinian thinking, so apparently the two do not match that well.
- Novacek (1986) presented the first cladistic analysis and removed the menotyphlan groups like elephant shrews and tree shrews. He included in the superorder Insectivora the two orders Leptictida and Lipotyphla and endorsed the division of the latter into the suborders Erinaceomorpha and Soricomorpha (also including tenrecs and golden moles).
- McKenna & Bell (1997) agreed, but preferred the name Lipotyphla for the grandorder Insectivora, and divided them in the three orders Chrysochloridea, Erinaceomorpha (redefined to include Talpidae), and Soricomorpha (incl. tenrecs but not golden moles).
- Molecular data increasingly suggested that Lipotyphla and Soricomorpha (incl. tenrecs and golden moles) are polphyletic (Madsen et al. 2001, Mouchaty et al. 2000a, 2000b, Murphy et al. 2001a, Springer et al. 1997, Stanhope et al. 1998a, 1998b).
- Stanhope et al. (1998a) finally suggested a new order Afrosoricida for tenrecs and golden moles within a clade of African mammals called Afrotheria.
- Waddel et al. (1999) therefore suggested a new order Eulipotyphla for moles, hedgehogs, and shrews. Indeed these authors did not really bother to formally establish this new taxon but just used the name twice in their publication, which is a remarkably “humble” origin for a new ordinal name among living mammals. The monophyly of this group and its position in Laurasiatheria was supported by multiple studies (Madsen et al. 2001, Malia et al. 2002, Murphy et al. 2001a).
Thus, Insectivora represents an obsolete systematic group, which united all kinds of insectivorous placental mammals, as different as pigs, aardvarks, flying lemurs (colugos), tree shrews, elephant shrews, golden moles, tenrecs, hedgehogs, moles, and true shrews. Insectivora also became a waste basket (Simpson 1945, Butler 1977, McKenna 1969, McKenna & Bell 1997, Asher 1999, Symonds 2005, Rose 2006a, Prothero 2017) for recent and fossil insectivorous mammal groups of uncertain affinity and status like for example Apatotheria (Apatemyidae), Cimolesta, Deltatheridia, Didymoconida, Leptictida, Mixodectoidea, Pantolestoidea, Palaeoryctoidea, and Proteutheria (e.g., Matthew & Granger 1915, Romer 1966, Van Valen 1966, 1967, 1978, Szalay 1969, 1977, Novacek 1976, Bown & Schankler 1982, Wyss 1987, Lopatin 2006, Gunnell & Bloch 2008). Van Valen (1966) already concluded that “the various groups of insectivores are placed in the same order mainly because they have not evolved sufficiently divergent overall specializations from the ancestral placentals to warrant our creating separate orders for them.”
Insectivores were considered to be the “ancestral stock” from which all other placental mammal orders derived (Huxley 1880, Matthew 1909, Wyss 1987). Similarly, Douady & Douzery (2003) mentioned that “‘Insectivores’ are one of the key groups in understanding mammalian origins,” which was also supported by morphological studies that initially suggested a basal position of lipotyphlan insectivores in the placental tree of life (e.g., Shoshani & McKenna 1998, Liu & Miyamoto 1999, Springer et al. 2004). Other experts rather believed in a close relationship of insectivores and primates (e.g., McKenna 1963 and Szalay 1969). Some earlier phylogenetic analyses came to very different results, such as the biomolecular study of Miyamoto & Goodman (1986), which suggested a closest relationship of insectivores with pangolins and carnivores. Most modern studies have different groups of insectivores redistributed among the three different cohorts Afrotheria, Euarchontoglires, and Laurasiatheria. Even though all these studies were based on Darwinian assumptions and extensive data, they still failed to agree on a single true tree of life, which is quite telling.
Menotyphla and Lipotyphla: An Obsolete Distinction
As we have seen above, the insectivore mammals were for a long time differentiated into the two subgroups Menotyphla and Lipotyphla. Menotyphla originally included flying lemurs or colugos (Dermoptera), tree shrews (Scandentia), and elephant shrews (Macroscelidea). Lipotyphla included the Erinaceomorpha (Erinaceidae) and Soricomorpha (Talpidae and Soricidae) and the Caribbean solenodons, as well as the Afro-Malagasy tenrecs and golden moles. The latter three groups were most often included in Soricomorpha (e.g., McKenna 1975), but sometimes separated as Zalambdodonta, or as Tenrecomorpha / Tenrecoidea and Chrysochloromorpha / Chrysochloroidea. Menotyphla became successfully disassembled, by first removing Dermoptera and Scandentia that were transferred to Archonta or even Primates (see Bechly 2023b), and finally Macroscelidea that were first transferred to Anagalida / Glires (e.g., McKenna & Bell 1997) and later to Afrotheria (Madsen et al. 2001; also see Bechly 2022). This left Insectivora to only include Lipotyphla, so that the two taxa became synonymous. Since Insectivora had this “ugly” history as artificial waste basket taxon for all kinds of small insect-eating mammals with elongate snouts and plantigrade pentadactyl feet (Gunnell & Bloch 2008), most later experts preferred to abandon Insectivora and rather use the name Lipotyphla (e.g., McDowell 1958, Butler 1972, and McKenna & Bell 1997) with Novacek (1976, 1992), Rose (2006), and Missiaen & Smith (2008) being a notable exceptions. The last relict usages that I managed to find for Insectivora as a formal mammalian order-group taxon in the modern technical literature were Meehan & Martin (2010), Gutierrez et al. (2020), and the NHB Atlas of European Mammals (2023).
The Polyphyly of Lipotyphla: Eulipotyphla and Afrosoricida
The monophyly of Lipotyphla was supported by various morphological characters (Butler 1956, 1972, 1988, McDowell 1958, Van Valen 1967, MacPhee & Novacek 1993), but some studies did not support a monophyletic Tenrecomorpha (e.g., MacPhee & Novacek 1993). In the late 1990s doubts began to grow about the monophyly of Lipotyphla (e.g., Piccinini et al. 1991, Springer et al. 1997, Emerson et al. 1999), especially after the seminal work of Stanhope et al. (1998a), who argued that tenrecs and golden moles are more closely related to an African clade (modern Afrotheria) than to other lipotyphlans, which was supported by Madsen et al. (2001), Malia et al. (2002), and Nikaido et al. (2003a). Not all the experts agreed, at least not initially: For example, Shoshani & McKenna (1998) compared the evidence from morphological and molecular data and still tentatively supported the monophyly of Lipotyphla, even though they did not rule out the possibility that Chrysochloroidea is more closely related to paenungulates (Afrotheria). Asher (1999) likewise found no support for a monophyletic Tenrecomorpha or an African clade, but nested Chrysochloridae and Tenrecidae at different places within Lipotyphla, similar to McKenna & Bell (1997). Asher (2001) added many soft-tissue characters but found a lot of homoplasy for either alternative (monophyletic Lipotyphla vs Afrotheria), even though the new soft-tissue characters rather agreed with previous osteological and dental evidence in favor of a monophyletic Lipotyphla. The most important support for Lipotyphla came from the supertree analysis by Liu et al. (2001), which even years later still represented the “by far most comprehensive higher-level phylogeny of placentals” (Beck et al. 2006). Liu & Miyamoto (1999) had already concluded that the inclusion of Chrysochloridae and Tenrecidae is the weakest part of the Afrotheria hypothesis. Corneli (2002) found significant support for Tenrecomorpha grouping with Lipotyphla based on complete mitochondrial genomes, but dismissed such mtDNA data as inadequate. Asher et al. (2003) found that the analysis of an expanded morphological data set alone weakly favored Lipotyphla rather than Afrotheria. Lopatin (2006) likewise supported Lipotyphla and included Tenrecomorpha in Soricomorpha and used the name Soricota for the Soricomorpha s.str. Rose (2006) discussed the pro and con arguments and concluded that the composition of Lipotyphla remains equivocal, so that he tentatively retained Lipotyphla as order within the superorder Insectivora. He also commented that “so far, the proposed order Afrotheria has been recognized, albeit strongly, solely from molecular evidence; no compelling anatomical evidence supporting this supposed clade has been identified”. Nevertheless, numerous modern phylogenomic studies supported the polyphyly of the traditional Lipotyphla (e.g., Waddell et al. 1999, 2001, Madsen et al. 2001, Murphy et al. 2001a, 2001b, 2007, Malia et al. 2002, Douady et al. 2002, Douady & Douzery 2003, Archibald 2003, Nikaido et al. 2003a, 2003b, Asher et al. 2005, Beck et al. 2006, Bininda-Emonds 2007, Gunnell & Bloch 2008, Prasad et al. 2008, Song et al. 2012, O’Leary et al. 2013, Halliday et al. 2015, Foley et al. 2016, Upham et al. 2019, Zachos 2020, Brady & Springer 2021).
Douady et al. (2002) commented:
Wagner (1855) included hedgehogs, moles, shrews, solenodons, golden moles, tenrecs, flying lemurs, tree shrews, and elephant shrews in Insectivora. Subsequently, morphologists excluded flying lemurs, tree shrews, and elephant shrews from Insectivora and placed these taxa in the orders Dermoptera, Scandentia, and Macroscelidea, respectively. The remaining insectivores constitute Lipotyphla, which is monophyletic based on morphology. In contrast, molecular data suggest that lipotyphlans are polyphyletic, with golden moles and tenrecs placed in their own order (Afrosoricida) in the superordinal group Afrotheria. Studies based on nuclear genes support the monophyly of the remaining lipotyphlans (= Eulipotyphla) whereas mitochondrial genome studies dissociate hedgehogs from moles and place the former as the first offshoot on the placental tree.
However, they also closed their article with the following remarkable admission:
… Afrotheria is now a superordinal clade for which the molecular evidence is so overwhelming … that it defies any possibility of reasonable refutation … and yet, after over 100 years of morphological investigation, there is not a single identified morphological synapomorphy for this clade.
Let this sink in: in the modern phylogenetic system, one of the four supergroups (cohorts) of placental mammals is not supported by any anatomical similarities! One really has to be willfully ignorant not to recognize this as a striking contradiction to any Darwinian expectations.
Erinaceomorpha and Soricomorpha: Another Obsolete Distinction
The above mentioned division of Lipotyphla into Erinaceomorpha and Soricomorpha (= Soricota sensu Kalandaze & Rautian 1992 and Lopatin 2006) was widely accepted among the experts (Gregory 1910, McDowell 1958, Butler 1956, 1972, 1988, McKenna 1975, Russell et al. 1975, Szalay 1977, Rose 1981, Novacek et al. 1985, Hooker 1986, Novacek et al. 1985, Novacek 1986, Carroll 1988, MacPhee et al. 1988, MacPhee & Novacek 1993, Storch 1996, McKenna & Bell 1997, Shoshani & McKenna 1998, Arnason & Jahnke 2002, Arnason et al. 2002, Douady et al. 2002, Smith et al. 2002, Kielan-Jaworowska et al. 2004, Tabuce et al. 2005, Lopatin 2006, Scott 2006, Gunnell & Bloch 2008, Gunnell et al. 2008, Secord 2008, Dunn & Rasmussen 2009, Silcox et al. 2010, Rose et al. 2012), with golden moles and/or tenrecs (Tenrecidae = Centetidae) mostly included in Soricomorpha, but sometimes separated as Tenrecomorpha (Butler 1972, Novacek 1976) and Chrysochloroidea, or united (sometimes with solenodons) as suborder Zalambdodonta (e.g., Gill 1884-1885, Matthew 1909, Gregory 1910, Van Valen 1967, Thenius 1969, Szalay 1977, Carroll 1988, Piccinini et al. 1991).
Hutterer (2005a) commented in the online edition of Wilson & Reeder (2005) on Erinaceomorpha:
Formerly included in the Insectivora (as in the last edition; Hutterer, 1993a) or Lipotyphla, but treated here as a separate order in consequence of the obvious paraphyletic nature of the Insectivora clade (Asher et al., 2002; Stanhope et al., 1998). Various genetic studies (Emerson et al., 1999; Liu et al., 2001; Mouchaty et al., 2000a, b; Nikaido et al., 2001) demonstrated that hedgehogs and soricomorphs keep distant positions in phylogenetic trees. Such results reflect ideas earlier expressed by paleontologists (Butler, 1988; McKenna, 1975) and are corroborated by a careful study of the morphology and relationships of fossil and extant zalambdodont mammals by Asher et al. (2002). The name Erinaceomorpha was proposed by Gregory (1910) and has since been widely used in the paleontological literature. It is adopted here in the sense of McKenna (1975) and Butler (1988). MacPhee and Novacek (1993) used it as a name for a suborder of Lipotyphla of unresolved relationships to other clades such as soricomorphs and chrysochloromorphs.
Hutterer (2005b) commented in the online edition of Wilson & Reeder (2005) on Soricomorpha:
Commonly included in the Insectivora (as in the last edition; Hutterer, 1993a) or Lipotyphla, but provisonally treated here as a separate order because of accumulating evidence for the paraphyletic nature of the former Insectivora clade (Asher, 1999, 2001; Stanhope et al., 1998). Various genetic studies (Emerson et al., 1999; Liu et al., 2001; Malia et al., 2002; Mouchaty et al., 2000a, b; Nikaido et al., 2001) demonstrated that soricomophs and hedgehogs, sometimes also moles, keep distant positions in phylogenetic trees. Such results are reflected by ideas earlier expressed by Butler (1988) and McKenna (1975), and are corroborated by the careful study of fossil and extant zalambdodont mammals by Asher et al. (2002). The name Soricomorpha was proposed by Gregory (1910) and has since been widely used in the paleontological literature. It is adopted here in the sense of McKenna (1975) and Butler (1988). MacPhee and Novacek (1993) used it as a name for a suborder of Lipotyphla of unresolved relationships to other clades such as erinaceomorphs (now Erinaceomorpha) and chrysochloromorphs (now Tenrecomorpha or Afrosoricida). The results of Stanhope et al. (1998) offer weak support for a relationship between “mole, shrew and solenodon”, e.g., Soricomorpha in the sense applied here. Other authors, however, suggest that even this clade may be polyphyletic. Malia et al. (2003) constructed a consensus tree for a set of 47 mammalian taxa including Sorex, Talpa, Scalopus, and Erinaceus. The shrews clustered with the bats, while the two moles and the hedgehog formed a separate trichotomy. Corneli (2002), who compared complete mitochondrial genomes, found that moles sister shrews and that hedgehogs are distantly related to both, which is in accordance with the Soricomorpha/Erinaceomorpha concept adopted here. Lin et al. (2002a) studied four mitochondrial genomes and found some support for a mole-shrew-hedgehog clade. Waddell et al. (1999) called this group Eulipotyphla. A further problem is the allocation of Solenodon and Nesophontes. Emerson et al. (1999) compared a 12 S rRNA sequence of Solenodon with various mammals and in a strict consensus tree placed the genus next to myomorph rodents. On the other hand, Asher (1999), Whidden and Asher (2001), and Asher et al. (2002) discussed possible relationships of Nesophontes and Solenodon to tenrecomorphs. At this stage there exist many conflicting views and no consistent phylogeny of the members of the former Insectivora.
The removal of the Tenrecomorpha (tenrecs, otter shrews, and golden moles) and their transfer to the afrotherian clade only left Erinaceomorpha and Soricomorpha in the order Lipotyphla. To distinguish this clade from previous artificial concepts of Lipotyphla, Waddell et al. (1999) suggested to abandon Lipotyphla just like the obsolete name Insectivora, and to use Eulipotyphla as new ordinal name for this clade. This suggestion was quickly adopted by other experts (e.g., Murphy et al. 2001a, 2001b, Waddell et al. 2001, Douady et al. 2002, Douady & Douzery 2003, Kemp 2005, Beck et al. 2006, Rose 2006a, Bininda-Emonds 2007, Springer et al. 2004, 2007a, 2007b, 2017, Wible et al. 2007, Prasad et al. 2008, Archer et al. 2010, Meredith et al. 2011, Rose et al. 2012, Song et al. 2012, Halliday et al. 2015, Manz et al. 2015, Foley et al. 2016, Esselstyn et al. 2017, Upham et al. 2019, Brady & Springer 2021) with only few dissenters, who advocated to keep the name Lipotyphla for the redefined clade (e.g., Archibald 2003, Asher et al. 2005, 2009, Gunnell et al. 2008, Asher & Helgen 2010, O’Leary et al. 2013, Vianey-Liaud et al. 2014, Crowell et al. 2024; also see Zachos 2020).
Monophyly vs Polyphyly of Eulipotyphla
However, this is not the end of the story, because even the monophyly of Eulipotyphla has been disputed and its diphyly suggested mainly based on mitogenomic analyses. These studies either suggested hedgehogs representing a basal branch of placental mammals (Cao et al. 2000, Arnason & Jahnke 2002, Arnason et al. 2002, Mouchaty et al. 2000a, 2000b; also see Krettek et al. 1995) or the sister group of all other Laurasiatheria (Kjer & Honeycutt 2007), while Soricomorpha belongs within Laurasiatheria and Tenrecomorpha within an African clade (Afrotheria). This basically agrees with the previous phylogenomic studies by Stanhope et al. (1998a) and Emerson et al. (1999) as well as the studies by Nikaido et al. (2001, 2003a). It also resonates with the remark by Novacek et al. (1985) that “erinaceomorph insectivores are a critical group for understanding eutherian phylogeny. Erinaceomorphs have been variously cited as ancestors or close relatives of tupaiids, primates, bats, dermopterans, and several other major eutherian taxa.” Nevertheless, such polyphyletic views were not confirmed by other subsequent studies, which increasingly and consistently supported the monophyly of Eulipotyphla (e.g., Nikaido et al. 2003b), with erinaceomorphs as a derived subgroup deeply nested within Eutheria, so that Eulipotyphla is generally still accepted as valid mammalian order to this day.
The Shifting Position of Eulipotyphla in the Mammalian Tree of Life
So, now that all the smoke has settled, everything is surely fine with Eulipotyphla, no? You will hardly be surprised that the answer is indeed, no. While the general composition of Eulipotyphla seems mostly settled indeed, the interordinal relationships of Eulipotyphla with other orders within Laurasiatheria is still quite controversial, even though most studies either have Eulipotyphla as most basal branch (e.g., Murphy et al. 2001a, 2001b, Meredith et al. 2011, Song et al. 2012, Esselstyn et al. 2017, Springer et al. 2004, 2007a, 2007b, 2017, Scornavacca et al. 2019) or more rarely as sister group of bats (e.g., Hooker 2014; also see Mouchaty et al. 2000b). However, the relationships of the laurasiatherian mammal orders are a mess and perfectly illustrate the general problem with phylogenetics. Hu et al. (2012) reviewed the phylogeny of Laurasiatheria and showed that for the relationships of the six orders not less than 16 (!) different tree topologies have been suggested by phylogenetic studies between 1999 and 2012 (see below for the reproduced figure 1 of Hu et al. 2012).
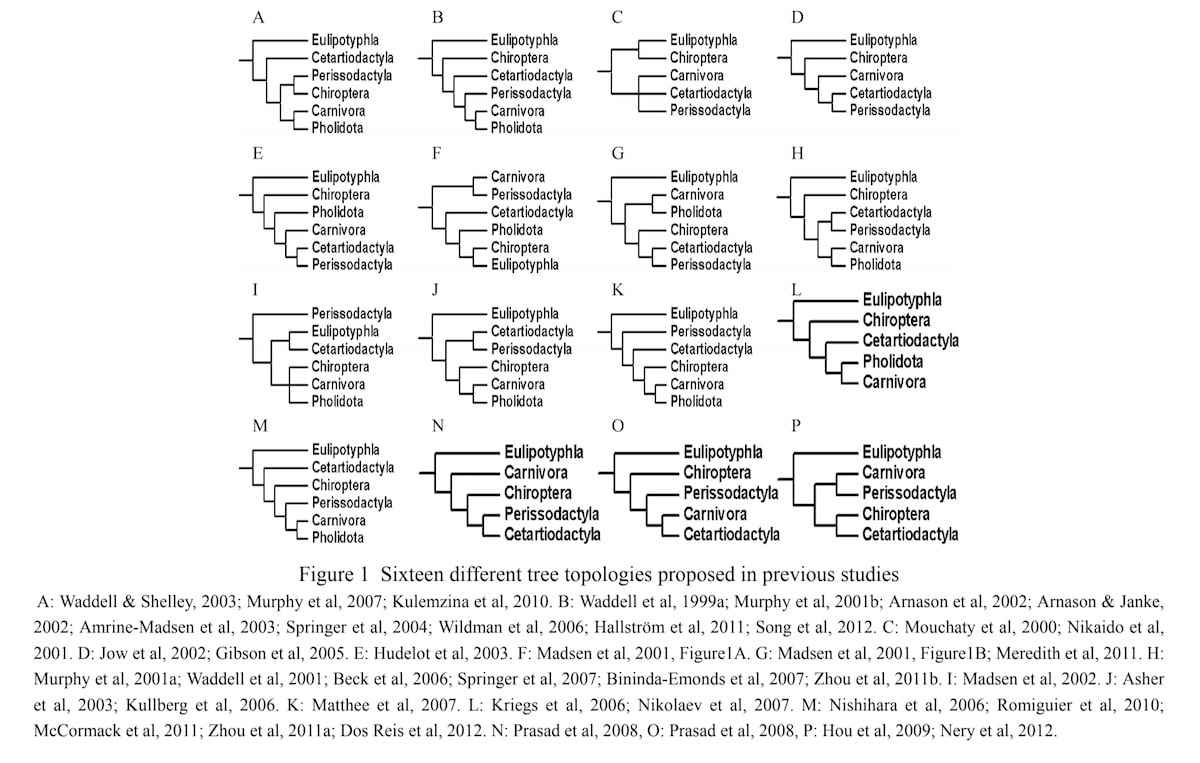
Most of these studies used hundreds of morphological characters and/or hundreds or even thousands of genes to reconstruct the phylogenetic trees, and still no consensus at all was reached. It is quite telling to find such a revealing figure not published in top-tier journals like Nature, where it should be a cover feature, but only well hidden in an obscure Chinese journal (also see Asher et al. 2009 published in low-impact journal BioEssays). The obvious reason is, that this inconvenient truth is not just conflicting with Darwinian expectations but can be considered as strong empirical evidence that something is fundamentally wrong with the theory. Different lines of data do not converge to a single true tree of life (also see Prasad et al. 2008: fig. 3), which means that morphological and genetic similarity is not a reliable indicator of common descent (even if common descent should be true, which is still my preferred view), against all the Darwinist claims to the contrary. This is a huge problem that evolutionary biologists will-fully ignore since several decades, and the scientific community happily lets them get away with this unscientific behavior, because otherwise their materialist bias and grandiose claims to fully explain the world “without a divine foot in the door” might be seriously challenged.
In a previous article at Evolution News (Bechly 2023d) I demonstrated with the example of arachnid arthropods that different published phylogenetic trees are so incongruent that their consensus would collapse into an unresolved polytomy, which contradicts the predictions from Darwinian evolutionary theory of common descent with modification. Insectivore mammals are yet another example for this severe problem. The review of the history of phylogenetic research on insectivores by Symonds (2005) illustrates the highly incongruent published trees that hardly have any common denominator apart from all being attributed to mammals. This applies to the relationship between insectivore orders as well as their relationship to other mammalian orders.
Controversial Relationships Between Living Eulipotyphlans
There has also been considerable disagreement about the intraordinal relationships of the four living families of Eulipotyphla (see Symonds 2005). Biltueva & Vorobieva (2012) therefore commented:
The sister-group relationships at the family level within this order have always been strongly debated [e.g. Symonds, 2005]. According to morphological data, there is a fundamental split into Erinaceomorpha and Soricomorpha, and shrews are grouped with moles [Butler, 1988]. In contrast, most molecular phylogenies support grouping shrews with hedgehogs to the exclusion of moles [Miyamoto and Goodman, 1986; Douady et al., 2002].
McKenna & Bell (1997) had resurrected the Erinaceomorpha sensu McDowell (1958) and Van Valen (1967), thus a grouping of hedgehogs and moles, without explicit basis (Douady et al. 2002). Such a relationship was also supported by the cladistic study of Schwermann et al. (2019).
Even though the traditional view from morphology rather grouped shrews with moles in the taxon Soricomorpha (e.g., Gregory 1910, Campbell 1939, Butler 1972, 1988), which was also strongly supported by the extensive supertree analyses of Liu et al. (2001) and Grenyer & Purvis (2003), the general consensus today agrees with modern phylogenomic studies that rather suggested a closer relationship of hedgehogs with shrews (e.g., Murphy et al. 2001a, 2001b, 2007, Douady & Douzery 2003, Asher et al. 2003, 2005, 2009, Roca et al. 2004, Beck et al. 2006, Dubey et al. 2007, Springer et al. 2007a, 2007b, 2017, Gunnell & Bloch 2008, Archer et al. 2010, Brace et al. 2016, He et al. 2016, Esselstyn et al. 2017, Casewell et al. 2019, Brady & Springer 2021).
Furthermore, a variety of studies suggested that Solenodontidae do not cluster with Soricomorpha, but together with the extinct Nesophontidae represent a more basal branch of Eulipotyphla (e.g., Beck et al. 2006, Brace et al. 2016, Springer et al. 2018, Casewell et al. 2019), which was named Solenodonta by Brace et al. (2016) (not to be confused with the group Solenodonta sensu Kalandadze & Rautian, 1992 for Apternodontidae+Solenodontidae). This suggests the following relationship for the recent eulipotyphlan families:
(Solenodontidae+Nesophontidae)+(Talpidae+(Erinaceidae+Soricidae))
A clade of hedgehogs and shrews was also supported by the chromosome study of Biltueva & Vorobieva (2012). O’Leary et al. (2013) only differed in recovering Talpidae even more basal than Solenodontidae, while Sato et al. (2019) recovered Talpidae as sister group of Solenodontidae. With tenrecs, solenodons, and talpids shown to be not forming a clade with shrews, the Soricomorpha turned out to be a highly polyphyletic group, so that all attributions of fossil taxa to Soricomorpha (see below) have to be considered as dubious or even obsolete. Also, it is remarkable that all three possibilities for a relationship of hedgehogs, moles, and shrews were advocated by eminent experts with a significant disagreement between morphological and molecular data. It is also hardly necessary anymore to mention that the stratigraphic distribution of the fossil record does not agree with the suggested phylogenetic relationships because the earliest hedgehogs are 61.7 million years old while the earliest moles are only 37.2 million years old.
An Up-to-Date Classification of Eulipotyphla
Anyway, I here follow the current (highly controversial) consensus and present this up-to-date classification of higher taxa in Eulipotyphla (Novacek et al. 1985, McKenna & Bell 1997, Lopatin 2006) with their stratigraphic ranges and oldest fossil records (extinct fossil taxa marked with +; datings mostly according to PaleoDB):
Eulipotyphla (66.043-0 mya)
+Adunator sp. ? (61.7-56.8 mya)
+Chambilestidae (not Chambelestidae) ? (55.8-40.4 mya)
+Micropternodontidae (46.2-20.43 mya)
Solenodonta
+Geolabididae? (= Centetodontidae) (63.3-15.97 mya)
+Stilpnodon simplicidens (63.3-61.7 mya)
+Nesophontidae (0.012-0 mya)
+Apternodontidae (61.7-33,3 mya)
+Asiapternodontinae (48.6-37.2 mya)
+Parapternodontinae (= Parapternodontidae) (55.8-50.3 mya)
+Oligoryctinae (= Oligoryctidae, or in stem Eutheria?) (46.2-33.3 mya)
Erinaceota
Erinaceoidea (Erinaceomorpha s.str.)
+Adapisoricidae (58.7-46.2 mya)
+Amphilemuride (= Dormaaliidae) (55.8-33.9 mya)
+Hylomysoidinae (55.8-48.8 mya)
+Creotarsidae (61.7-46.2 mya)
+Diacodon ? (55.8-50.3 mya)
+Quadratodon sigei (66.043-61.7)
+Seia shahi (58.7-40.4 mya)
+Scenopaginae
+Sespedectinae
+Talpavoides dartoni (61.7-50.3 mya)
+Talpavus (50.3-37.2 mya)
+Vastanidae (55.8-48.6 mya)
Erinaceidae (61.7-0 mya)
+Auroralestes simpsoni (55.8-50.3 mya)
+Cedrocherus sp. (61.7-56.8 mya)
+Entomolestes sp. (61.7-37.2 mya)
+Litolestes ignotus (58.7-56.8 mya)
+Oncocherus krishtalkai (61.7-56.8 mya)
+Silvacola acares (55.8-50.3 mya)
+Brachyericinae (28.4-11.608 mya)
+Galericinae (48.6-4.2 mya)
+Taxon innom. (= Changlelestidae)
+Changlelestinae (48.6-23.03 mya)
+Tupaiodontinae (48.6-23.03 mya)
Erinaceinae (33.9-0 mya)
Hylomyinae (15.97-0 mya) (= Echinosoricinae)
Soricoidea (Soricomorpha s.str.)
+Nyctitheriidae (66.043/61.7-23.03 mya)
+“Amphidozotheriinae” (28.4-23.3 mya)
+“Asionyctinae” (55.8-48.6 mya)
+Eosoricodontinae (or in Chiroptera) (55.8-48.6 mya)
+“Nyctitheriinae” (66.043/61.7-28.4 mya)
+Placentidentinae? (or in Amphilemuridae) (55.8-40.4 mya)
+Praolestinae (55.8-48.6 mya)
+Talpilestinae (55.8-48.6 mya)
+Plesiosoricidae (55.8-8.7 mya)
+Ordolestes ordinatus (55.8-48.6 mya)
+Cretasorex arkhangelskyi (max. Late Miocene, not 94.3-89.3 mya!)
+Crocidosoricinae (28.4-3.2 mya)
+Heterosoricinae (40.4-7.75 mya)
+Limnoecinae (30.8-9.7 mya)
+Soricolestinae (48.6-37.2 mya)
+Soricolestes soricavus (48.6-37.2 mya)
Crocidurinae (15.97-0 mya)
Myosoricinae (= Crocidosoricinae?) (0 mya)
Soricinae (16.9-0 mya)
Talpoidea (37.2-0 mya)
+Dimylidae (23.3-7.75 mya)
+Dimylinae (23.3-8.07 mya)
+Proscalopidae (33.9-13.6 mya)
+Eotalpa anglica (37.2-33.9 mya)
+Oreotalpa florissantensis (37.2-33.9 mya)
+Suleimaninae (23.03-15.97 mya)
Desmaninae (= + Gaillardiinae)
Scalopinae (Upper Oligocene – 0 mya)
“Talpinae” (28.4-0 mya)
Uropsilinae (23.03-0 mya)
Insectivore-like basal (stem) Eutheria that were erroneously attributed by some scientists to Insectivora/Lipotyphla or even Eulipotyphla (also see this internet article by Jehle 2006 for a nice review of these mainly Paleocene groups):
+Adapisoriculidae? (66.043-40.4 mya)
+Adapisoriculus sp. (= Nycticonodon) (66.043-48.6 mya)
+Afrodon sp. (66.043-48.6 mya)
+Deccanolestes sp. (70.6-66.043 mya)
+Proremiculus lagnauxi (66.043-61.7 mya)
+Apatemyidae (= Apatotheria) (61.7-30.8 mya)
+Palaeoryctidae (=Palaeoryctida) (70,6-37.3 mya)
+Sarcodontidae (incl. Carnilestes Wang & Zhai 1995) (61.7-37.2 mya)
+Didymoconidae (61.7-15.97 mya)
+Gypsonictopidae (70.6-66.043 mya)
+Leptictidae (= Diacodontinae) (63.3-30.08 mya)
+Otlestidae (130-66.043 mya)
+Zalambdalestidae (94.3-61.7 mya)
+Zhelestidae (= Paranyctidae nomen nudum) (160.8-66.043 mya)
Below, I have included in alphabetical order some comments on the various fossil taxa listed in the classification above, to illustrate the stunning amount of uncertainty and controversy concerning their composition and relationships, which makes evolutionary classification look like a quite arbitrary endeavour. Decide for yourself.
Adapisoricidae
Adapisoricids are small insectivore mammals, which only include a single described species Adapisorex gaudryi from the Paleocene of France and some undetermined material from the Paleocene and Eocene of Europe and North America.
Gregory (1910) and Szalay (1975) suggested a closer relationship of Erinaceomorpha and especially Adapisoricidae with Primates, which was tentatively accepted by some scientists (e.g., Maier 1977, 1979) but thoroughly rejected by Novacek et al. (1983) and MacPhee et al. (1988).
Simpson (1928b) thought that Adapisoricidae may prove to be not distinct from Tupaiidae. Simpson (1945) included adapisoricids within Leptictidae in the insectivoran superfamily Erinaceoidea. Van Valen (1967, 1978) classified Adapisoricidae with the suborder Erinaceota of Insectivora, but suggested that Soricidae could have evolved from Plesiosoricidae or Adapisoricidae. Szalay (1968b) thought that they are “the stem erinaceoids (i.e.,the bulk of the genera referred to the Adapisoricidae by Van Valen, 1967)”. Many experts included Adapisoricidae in Erinaceomorpha (Butler 1972, Schmidt-Kittler 1973, Russell et al. 1975, Krishtalka 1976a, Novacek 1976, McKenna & Bell 1997, Lopatin 2006, and Rose 2006a), which was also supported by the first cladistic study of Novacek (1985). Novacek (1985) and Novacek et al. (1985) transferred most previous adapisoricid genera to Dormaaliidae and considered the type genus Adapisorex as an Erinaceidae, which was also endorsed by Carroll (1988).
Hooker (1986) considered Adapisoricidae as part of Erinaceomorpha and mentions only few differences between Adapisoricidae and Amphilemuridae. Indeed, some experts considered Adapisoricidae as just a synonym of Amphilemuridae (e.g., Thenius 1969). Similar to the latter author, Agusti & Antón (2002) considered Adapisoricidae as genuine insectivores in Erinaceomorpha, but also included Adapisoriculus and the amphilemurid Macrocranion in this family. A close association of adapisoricids and amphilemurids (dormaaliids) had previously been suggested (or implied) by Van Valen (1967), Russel et al. (1975), Kristalka (1976a), Bown & Schankler (1982), Gingerich (1983), Storch (1996), and Senekowitsch (2013).
However, all these attributions to Erinaceomorpha have to be considered as dubious, because more recent cladistic studies (e.g., Manz & Bloch 2015, Manz et al. 2015) suggest that the assemblage of extinct and living taxa usually included in Erinceomorpha do not seem to be a natural group. But it gets worse: another modern cladistic study by Hooker & Russell (2012) suggested that Adapisoricidae represent a paraphyletic or even polyphyletic assemblage of stem group Macroscelidea, thus have to be attributed to a different cohort than eulipotyphlans (Afrotheria instead of Laurasiatheria).
Adapisoriculidae
“Adapisoriculidae are an enigmatic group of small mammals known from the late Cretaceous of India, and from the early Palaeocene to early Eocene of Europe and Africa. Based on their primitive dental morphology, they have been classified as didelphids, nyctitheriids, leptictids, mixodectids, tupaiids, and palaeoryctids. While the latest hypothesis based on dental morphology suggests an affinity with Lipotyphla, postcranial remains indicate a close relationship with Euarchonta” (De Bast et al. 2012). According to the latter authors, the family includes seven genera and 16 species, ranging from mouse-sized to rat-sized, with long tails and possibly adapted for climbing in trees.
Smith et al. (2010) summarized that “the controversial family Adapisoriculidae, a group of shrew-sized Paleocene mammals, had proposed relationships with insectivores, marsupials and more recently to plesiadapiforms.” The Adapisoriculidae include some of the oldest putative crown group representatives of placental mammals such as Deccanolestes from the Late Cretaceous of India and Afrodon from the Paleocene of Africa. This makes Adapisoriculidae to an important group for understanding the origin and timing of the placental mammal radiation after the extinction of non-avian dinosaurs.
Matthew et al. (1910) included Adapisoriculus in Adapisoricidae, which was also still accepted by Szalay (1969). Simpson (1945) considered Adapisoriculus as a possible Didelphidae, thus as a marsupial opossum, but also listed the same genus as a possible insectivore of uncertain position. The family group taxon Adapisoriculinae was erected by Van Valen (1967) for Adapisoriculus, which he considered as a primitive tupaiid. Carroll (1988) listed Adapisoriculus as Insectivora incertae sedis. Following the opinion of McKenna & Bell (1997) and Gheerbrant & Russell (1989, 1991), Lopatin (2006) listed Adapisoriculidae with a question mark as most primitive member of Lipotyphla of uncertain relationship. A similar position was indicated by Rose (2006), who said that these “primitive eutherians … are probably, but not certainly, lipotyphlans”. Storch (2008) suggested a relationship with plesiadapiforms rather than lipotyphlan insectivores. Prasad (2009) accepted this and endorsed an euarchontan relationship. Based on tarsal and dental morphology, Smith et al. (2010) rejected an affinity of Adapisoriculidae with lipotyphlan insectivores, didelphid marsupials, or leptictids, in favor of an euarchontan affinity close to plesiadapiforms or to living colugos, which was rejected by Manz et al. (2015) as based on convergent adaptations to arboreality.
Seiffert (2010a) doubted all these placements and considered Adapisoriculidae as Placentalia incertae sedis or as very basal Afrotheria or even Afrosoricida. The latter position was mainly based on Afrodon (see Bechly 2023a), which was originally described as an insectivoran of the extinct family Adapisoriculidae (Gheerbrant 1988, Gheerbrant & Russell 1989, Gheerbrant et al. 1998). Later, it was found that Afrodon shares dental similarities with supposed early afrosoricids (Seiffert & Simons 2000, Seiffert et al. 2007). Asher & Seiffert (2010) and Seiffert (2010b) even recovered this genus as most basal stem Afrosoricida in their cladogram. However, Pickford et al. (2008) still considered Afrodon as an adapisoriculid insectivoran of the living mammal order Lipotyphla.
More recent comprehensive phylogenetic analyses (Wible et al. 2007, 2009, Goswami et al. 2011, Hooker 2014, Manz et al. 2015) strongly suggest that Adapisoriculidae (i.e., Deccanolestes) are very basal Eutheria, far outside the crown group of Placentalia. Indeed, they even seem to be more basal than other stem placentals such as Zhelestidae, Asioryctidae, Cimolestidae, Zalambdalestidae, and Leptictidae.
De Bast & Smith (2017) commented:
The systematic affinities of Adapisoriculidae has been very controversial: it has been identified as a lipotyphlan insectivore (Gheerbrant 1995), a stem primate (Storch 2008), a stem euarchontan (Smith et al. 2010), and an afrosoricid (Seiffert 2010). A recent phylogenetic analysis has suggested that the family could represent an arboreal group of basal eutherians (Goswami et al. 2011).
Therefore, De Bast & Smith (2017) tentatively classified Adapisoriculidae as of uncertain grandorder, thus explicitly not in Lipotyphla. However, they mentioned that the tarsal bones display derived homologies (synapomorphies) with Euarchonta (also see De Bast et al. 2012).
Even though Adapisoriculidae includes some of the oldest known placental mammals, we cannot use it to date the origin of Eulipotyphla because of the great uncertainty concerning the affinities of this family. This is also evident from the uncertain composition of the family Adapiosoriculidae. Here are two examples:
Nycticonodon Quinet, 1964 is listed as Soricomorpha without family assignment by PaleoDB, maybe based on Carroll’s (1988) listing as Soricoidea incertae sedis. However, according to Van Valen (1967) this genus name represents an invalid nomen nudum, and according to McKenna & Bell (1997: 272) it is an objective junior synonym of Adapisoriculus Lemoine, 1885.
The genus Garatherium from the Eocene of Algeria was originally descriped as a marsupial, which was accepted by McKenna & Bell (1997). Gheerbrant (1991, 1995) suggested adapisoriculid affinities within Lipotyphla, which was accepted by Rose (2006) and De Bast et al. (2012). Seiffert et al. (2010) considered this genus as a placental and possible stem tenrecoid among Afrotheria. Seifert (2010) even said that “Afrodon, Garatherium, “Palaeoryctes” minimus, Todralestes, Widanelfarasia, and possibly Chambilestes are stem or crown afrosoricids”, thus attributed at least three of the seven known genera of Adapisoriculidae to Afrotheria.
Adunator
The genus Adunator (= Mckennatherium) was a scansorial insectivore first described by Russell (1964). Meanwhile, nine species have been described from the Paleocene of the United States. Van Valen (1967) assigned this genus to the family Adapisoricidae within erinaceoid insectivores. Van Valen (1967) and Szalay (1969) considered Adunator as a leptictid. Novacek et al. (1985) and Butler (1988) also considered Adunator to be at the base of the erinaceomorph radiation, which was also accepted by Storch (1996) and McKenna & Bell (1997). Rose (2006) considered Adunator as a very primitive erinaceomorph. Carroll (1988), Gunnell et al. (2008) and Secord (2008) considered Adunator as an Erinaceomorpha of uncertain position. Eberle et al. (2014) mentioned Adunator as earliest erinaceomorph.
However, the cladistic study by Manz et al. (2015) recovered Adunator as sister group of Nyctitheriidae within Eulipotyphla, while Manz & Bloch (2015) found Adunator to be the sister group of Macrocranion (Amphilemuridae) near the base of nyctitheriid euliotyphlans. Either way Adunator would fall in Soricomorpha rather than Erinaceomorpha.
Benton et al. (2015) used Adunator ladae to date the minimum age of Eulipotyphla to 61.6 million years (also see Fossil Calibration Database), based on the attribution of Adunator to Erinaceomorpha by Novacek et al. (1985) and Smith et al. (2002) (also see Carroll 1988), and based on the dating of the top of the Danian to 61.6 mya by Woodburne (2004) and Gradstein et al. (2012). Therefore, Springer et al. (2017) still listed Adunator as oldest crown fossil of the Erinaceidae+Soricidae clade.
But this use of Adunator for calibrating molecular clock datings is hardly justified given the fact that its position in Eulipotyphla is not uncontroversial: Agusti & Antón (2002) listed Adunator among lepticids as an archaic eutherian, but did so without any source or explanation. After all, who cares about petty evidence in a book by Columbia University Press on 65 million years of mammalian evolution [sarcasm warning]. Anyway, much more serious is the fact that the more recent cladistic analysis by Hooker & Russell (2012) recovered Adunator as most basal branch in the “adapisoricid” stem group of Macroscelidea, thus placed in a different cohort (Afrotheria) than the laurasiatherian Eulipotyphla.
Amphilemuridae (= Dormaaliidae)
Amphilemuridae were small insectivore mammals from the Paleocene and Eocene of Europe and North America, which resembled hairy hedgehogs and some species like Macrocanion tenerum even had spines (Lehmann 2018), which are preserved in some of its numerous beautiful fossils from the famous Eocene Messel locality in Germany.
Amphilemurids were initially considered to be related to tree shrews or primitive primates (e.g., by Simpson 1945, who included Amphilemur in Adapidae), but most modern experts classified Amphilemuridae (or their synonym Dormaaliidae) as erinaceomorph eulipotyplans (Thenius 1969, Van Valen 1967, Bown & Schankler 1982, Novacek et al. 1985, Hooker 1986, Carroll 1988, MacPhee et al. 1988, Storch 1996, McKenna & Bell 1997, Smith et al. 2002, Lopatin 2006, Maitre et al. 2006, Rose 2006a, Gunnell & Bloch 2008, Beard & Dawson 2009, Dunn & Rasmussen 2009, Rose et al. 2012).
Van Valen (1967) even synonymized Amphilemuridae and Dormaaliidae with the subfamily Creotarsinae within Adapisoricidae. Russell et al. (1975) classified some genera like Macrocranion as subfamily Dormaaliinae in Adapisoricidae, which he considered as “close to the origin of the Erinaceidae“, while he retransferred the genus Amphilemur to Primates. This was reversed by Koenigswald & Storch (1983). Several works considered the family Amphilemuridae as a synonym of Adapisoricidae (e.g., Van Valen 1967, Thenius 1969). Agusti & Antón (2002) also listed Macrocranion as an adapisoricid. Wolfgang Maier, a German mammologist and former professor of mine at Tübingen University, also speculated that the genus Macrocanion (= Messelina) and adapisoricids could be closer related to primates (Maier 1977, 1979), while most other works included this genus in Amphilemuridae (e.g., Smith et al. 2002).
Differing from all previous studies that suggested an erinaceomorph relationship, the cladistic study of Manz et al. (2015) recovered Macrocranion (and thus Amphilemuridae) as paraphyletic towards Adunator + Nyctitheriidae, which implicitly suggests a soricomorph affinity. Manz & Bloch (2015) found a similar position near the base of Nyctitheriidae, but suggested a sister group relationship of Macrocranion and Adunator.
Another totally different view was proposed by Hooker & Russell (2012), who considered amphilemurids as stem Macroscelidea in the cohort Afrotheria (see Bechly 2022) instead of the cohort Laurasiatheria to which eulipotyphlans belong.
Even the composition of included genera is controversial: The example of Macrocranion was already discussed above. Another example is the genus Placentidens, which has been included in a separate subfamily within Amphilemuridae (McKenna & Bell 1997), or classified in Nyctitheriidae (Beard & Dawson 2009, Jones & Beard 2023).
Apatemyidae (Apatotheria)
Apatemyidae were small insectivorous mammals from the Paleogene of North America, Europe and India, which were specialized for life in the trees. Some like the genus Heterohyus had very strange adaptations with elongated fingers like the living Malagasy aye-aye (Koenigswald 1987, Kalthoff et al. 2004), or looked like saber-toothed shrews (Prothero 2017).
They were established as family by Matthew (1909) and considered as Insectivora of uncertain position (“very doubtfully referred to the Insectivora”). Based on an early suggestion of Scott & Jepson (1936) (also see McKenna 1963), McKenna (1975) established for apatomyids a separated mammal order Apatotheria, which was accepted by many subsequent workers (e.g., Sigé 1975, Russell et al. 1979, Hooker 1986, Carroll 1988, Silcox et al. 2010).
Until the mid 20th century, apatemyids were mostly considered to be closely related to the plesiadapids and thus primates or associated with the rodent-like mixodectids in Proglires (see historical review in McKenna 1963). Simpson (1945) classified Apatemyidae as prosimian primates of uncertain infraordinal relationship. Robinson (1966) considered Apatemyidae as of uncertain position in primates or insectivores. Szalay (1969) only made the very vague statement that “although apatemyids are phylogenetically non-primates, they show remarkable trends that may resemble the insectivore-primate transition.”
Many authors considered Apatemyidae as Insectivora (McKenna 1963, Thenius 1969, Gingerich & Rose 1982, Koenigswald 1987, 1990, and Gunnell 1989). Others considered Apatemyidae as Proteutheria among insectivoran mammals (Van Valen 1967, Butler 1972, Novacek 1976, Rose 1981, and Bown & Schankler 1982).
McKenna (1975) associated Apatemyidae and Carnivora within the grandorder Ferae, as previously suggested by Lillegraven (1969). Hooker (1986) likewise said that “its position herein adjacent to the Carnivora and distant from the Proteutheria reflects its relationships with the palaeoryctids as advocated by Szalay (1968[b])”. McKenna & Bell (1997) classified Apatotheria as a suborder of Cimolesta in Ferae, together with living pangolins and carnivores, which was accepted by some later works (e.g., Secord 2008). Mainly based on McKenna & Bell (1997), Lopatin (2006), Rose (2006) still classified Apatotheria in the superorder Ferae, but also mentioned that “their relationships have been variously considered to lie with “proteutherians,” Plesiadapiformes, or Cimolesta, but none of these proposals has been convincingly demonstrated”. Beard & Dawson (2009) classified Apatemyidae as a family in the extinct Cimolesta, a carnivorous order of basal eutherians.
Agusti & Antón (2002) considered Apatemyidae as a group of archaic placental mammals and not closely related to living hedgehogs, shrews, and moles. Kemp (2005) thought they might possibly be highly derived palaeoryctids, but considered their relationship as obscure. The analysis of Silcox et al. (2010) revovered them as basal members of the cohort Euarchontoglires. An outlier seems to be the study of Halliday et al. (2015), who found Apatemys as sister group to bats (Chiroptera). The most recent cladistic study by Crowell et al. (2024) resolved Apatemyidae (Labidolemur) together with the Picrodontidae that were previously considered as plesiadapiform primates, as sister group of Glires.
Carroll (1988) summarized that “Apatemyids have been allied with ungulates, rodents, primates, carnivores, taeniodonts, tillodonts, condylarths, a variety of “insectivores,” and “proteutherians.”” and therefore listed them as eutherian mammals of uncertain position. Kalthoff et al. (2004) still said that “apatemyids are an extinct mammalian family lacking any related forms in the extant fauna”. This phylogenetic uncertainty is even more surprising given the fact that apatemyids are not just known by a few isolated teeth or bones, but by perfectly preserved complete skeletons even with preserved soft parts, body outline, and a tufted tail (e.g., Kalthoff et al. 2004, Jehle 2006). If biological similarity is mostly explained by common ancestry, then why is it, that even a large amount of data and experts cannot generate any agreement on the true phylogenetic relationships of such a group. This would be well explained if similarity is rather due to design and therefore not necessarily showing a pattern of nested hierarchies.
Apternodontidae and Parapternodontidae
Apternodontidae is a family of small insectivore mammals from the Paleogene of North America and East Asia, which shares with the Caribbean solenodons and the African golden moles and tenrecs a zalambdodont dentition named after a V-shaped pair of ridges on the upper molar crowns that resembles the Greek letter lambda (note that according to Asher et al. 2002 the fossil Zalambdalestidae, in spite of their name, had no proper zalambdodont dentition). Van Valen (1967) even included Apternodontinae as subfamily of Tenrecidae. According to Dawson & Krishtalka (1984) apternodontids include the ancestors of the zalambdodont living insectivores, i.e. tenrecs, golden moles, and solendons. Sometimes these animals were united in a distinct insectivore mammal order Zalambdodonta (e.g., Matthew 1909 and Thenius 1969).
Most often all the living taxa were included in Lipotyphla, so that apternodontids were very early associated with lipotyphlan insectivores (e.g., Jaekel 1911). In spite of the zalambdodont dentition, McDowell (1958) rejected a relationship of this family with Lipotyphla and rather suggested a possible creodont affinity. Some subsequent studies considered Apternodontidae as soricomorph insectivorans (McKenna 1975, Novacek 1976, Bown & Schankler 1982, Gunnell & Bloch 2008), and McKenna & Bell (1997) even included them in Soricoidea. Likewise, Carroll (1988) thought the Apternodontidae are primitive insectivores that “may have risen from, or in common with, the Geolabididae” within Soricoidea, and he listed Parapternodus as Soricoidea incertae sedis. McDowell (1958) had excluded Apternodus from Soricomorpha and even from Lipotyphla (Insectivora). Robinson & Kron (1998) again classified Apternodontidae in Insectivora.
The most thorough study of Apternodontidae was provided by Asher et al. (2002), who also described the new families Oligoryctidae and Parapternodontidae for genera previously classified in Apternodontidae. Asher et al. (2002, 2005) and Archer et al. (2010) recovered Apternodontidae, Oligoryctidae and Parapternodontidae as successive sister groups of Soricidae, which contradicts the classification of these groups as subfamilies of Apternodontidae and their previously suggested relationship with the living West Indian solenodons (e.g., Simpson 1945).
Nevertheless, Lopatin (2003a) suggested that within Soricomorpha a close relationship of Apternodontidae and living Solendontidae is not only supported by the similar zalambdodont dentition but also by the cranial structure. Therefore, Lopatin (2006), in spite of Asher et al (2002), united Apternodontidae with Solenodontidae in a common superfamily Solenodontoidea (= Solenodonta sensu Kalandadze & Rautian 1992). On the other hand, the cladistic study of Gunnell (2008: fig. 7.2) found a clade of Apternodontidae, Parapternodontidae, and Oligoryctidae within Soricoidea, thus not a paraphyletic grade towards Soricidae as proposed by Asher et al. (2002). Rose (2006) and Rose et al. (2012) listed Parapternodontidae within eulipotyphlan Soricomorpha, and mentioned concerning the previous grouping with solenodons that “this grouping seems to be based largely on zalambdodonty (a condition known to have arisen multiple times) and perhaps the occurrence of both genera in the New World” (Rose 2006a). It looks like the views among experts still fluctuate between a solenodontid and a soricid affinity.
The oldest fossil record seems to be an undetermined apternodontid from the Paleocene (61.7-56.8 mya) Polecat Bench Formation of Wyoming (see PaleoDB).
Chambilestidae
Chambilestes foussanensis was described by Gheerbrant & Hartenberger (1999) from a jaw fragment from the Lower Eocene of Tunesia and assigned to a distinct new family of archaic insectivores. The authors suggested a close relationship with Laurasian erinaceomorph lipotyphlans, but only with a question mark. Rose (2006) classified Chambilestidae as Erinaceomorpha but said in a footnote that it “may belong in Soricomorpha”. Lopatin (2006) also listed Chambilestidae as primitive Lipotyphla of uncertain relationship with a question mark. Seiffert (2010a) mentioned that the phylogenetic analysis of Gheerbrant & Hartenberger (1999) did not support their suggestion of an erinaceomorph relationship. His own cladistic study placed Chambilestidae in a very basal position of Afrotheria, thus in a different cohort than lipotyphlan insectivores. Seiffert (2010a) also mentioned that “possibly Chambilestes are stem or crown afrosoricids”.
Changlelestidae
Changlelestidae were first described by Tong & Wang (1993) from the Early Eocene of Shandong in China and attributed to soricomorph insectivores. Beard & Dawson (1998) agreed and considered Changlestidae as closely related to Nyctitheriidae and/or with Plesiosoricidae and Soricidae. Tong and Wang recognized the dental similarity to the erinaceomorph Tupaiodon and considered them as potential member of the same family. McKenna & Bell (1997) and Lopatin (2002, 2004) transferred Ernosorex to Plesiosoricidae, and considered the remaining changlelestines as earliest members of the erinaceomorph hedgehog subfamily Tupaiodontinae or at least as closely related (also see Gould 1995). Lopatin (2006) recognized Changlelestinae as a distinct subfamily of Erinaceidae, independent of Tupaiodontinae. The position of Tupaiodontinae in Erinaceidae seems to be well established (e.g., Gould 1995).
Creotarsidae
This Paleogene family only includes the North American genera Creotarsus and Xenacodon. It was placed in Creodonta incertae sedis by Simpson (1945), but has generally been attributed to Erinaceomorpha (e.g., Van Valen 1967, Novacek et al. 1985, McKenna & Bell 1997, Lopatin 2006, Rose 2006a). Van Valen (1967) considered Amphilemuridae and Dormaaliidae as synonyms of Creotarsinae that he classified as a subfamily of Adapisoricidae, which was rejected by Russell et al. (1975) but still tentatively accepted by Novacek (1976). Van Valen (1967) also speculated that some genera of Creotarsinae must be removed to the Erinaceidae. Bown & Schankler (1982) and Carroll (1988) listed Creotarsus as Insectivora of uncertain position, and Xenacodon as Erinaceidae.
Didymoconidae
Simpson (1945) included Didymoconus as Arctocyonoidea incertae sedis in the carnivore suborder Creodonta. Van Valen (1966, 1967) included this group in the superfamily Palaeoryctoidea of the insectivore order Deltatheridia, which was accepted by Thenius (1969). McKenna (1975) suggested a possible relationship with Anagalida, together with Zalambdalestidae. Szalay (1977) grouped it with hares and rabbits in Lagomorpha. Gingerich (1981) considered Didymoconidae as members of carnivorous Mesonychia (related to ungulates and whales) within Condylarthra. Carroll (1988) listed Didymoconidae as Mammalia incertae sedis. Meng et al. (1995) studied the cranial morphology and classified didymoconids as Insectivora, which was also endorsed by Wang et al. (1998), while their colleague Ting (1998) considered them as of uncertain order in the very same journal volume. McKenna & Bell (1997) included the family in Leptictida. Lopatin (2003b) summarized that “the Didymoconidae is a family of Paleogene Asian insectivores of uncertain phylogenetic relationships. Currently, the Didymoconidae are usually considered to be related to the Lipotyphla (Meng et al., 1994[sic]) or Leptictida (McKenna and Bell, 1997) or ranked as a separate order, the Didymoconida (Lopatin, 2001a), of the superorder Insectivora (sensu Novacek, 1986).” Lopatin (2006) included Lipotyphla, Leptictida, and Didymoconida as separate orders within a superorder Insectivora, while he attributed the order Cimolesta to the superorder Ferae. Rose (2006) listed the family among Cimolesta, apparently based on their shared carnivorous adaptations. This was also accepted by Missiaen & Smith (2008). Missiaen et al. (2013) still called it an “enigmatic group of Asian endemic insectivorous mammals” of which their study “did not resolve the higher level affinities”. Again, we see no visible progress in decades of research.
Dimylidae
Dimylids are only known from the Oligocene and Miocene of Europe. Several early works listed Dimylidae in Erinaceoidea or Erinaceomorpha (Simpson 1945, Van Valen 1967, Thenius 1969), and the general consensus since McKenna (1975) and McKenna & Bell (1997) seems to be that Dimylidae is related to moles within the superfamily Talpoidea (Lopatin 2006, Rose 2006a, Sánchez-Villagra et al. 2006). McDowell (1958) included Dimylidae and Talpidae in Erinaceomorpha, but mentioned that “is known almost entirely from teeth, and its affinities to the Lipotyphla are not proved”. Novacek (1976) and Novacek et al. (1985) only included Dimylidae in Erinaceomorpha with a question mark, because Schmidt-Kittler (1973) had questioned whether Dimylidae belongs to Erinaceomorpha or rather to Soricomorpha, but still as close relatives of moles. Therefore, McKenna (1975) assigned Dimylidae to Soricomorpha, and Carroll (1988) even to Soricoidea.
Galericinae
Galericinae were classified as a subfamily of the living hedgehog family Erinaceidae by Van Valen (1967) (also see Novacek 1985, McKenna & Bell 1997, Lopatin 2004a, 2006, Rose 2006a), or the genera were simply included in Erinaceidae (Carroll 1988). One author even listed Galericidae as a distinct family of hedgehog relatives (Zachos 2020). Often the name Galericinae is still used as synonym for the recent erinaceid subfamily Hylomyinae (e.g., Grenyer & Purvis 2003, Symonds 2005, Gunnell et al. 2008, Dunn & Rasmussen 2009, Kim et al. 2017; also see Wilson & Reeder 1993), which includes the living hairy hedgehogs or moonrats from South-East Asia. However, the relationship of these recent animals with the fossil genus Galerix is highly disputed, with some authors considering Galerix to be more basal in Erinaceidae than any living genera (e.g., Gould 1995). Also, Frost et al. (1991) argued that Galericinae does not meet the formal requirements for a nomenclatural priority. Therefore, it is definitely preferable to classify living moonrats in Hylomyinae and those fossil hedgehogs in a separate extinct subfamily Galericinae with Holarctic distribution from the Middle Eocene to the Early Pliocene. The earliest member of these Galericinae seems to be Eogalericius butleri from the Middle Eocene of Mongolia (Lopatin 2004a).
Geolabididae (= Centetodontidae)
Geolabididae is an extinct group of small shrew-like insectivores that were widespread in the Paleogene of North America, but also were recorded from the Upper Paleocene of Mongolia (Lopatin 2004b). The alleged Late Cretaceous genus Batodon has meanwhile been removed from Geolabididae and transferred to the basal eutherian Cimolestidae (but see Lopatin 2006 for an opposing view). Batodonoides vanhouteni from the Early Eocene of Wyoming is remarkable for representing the smallest mammal ever known (Bloch et al. 1998, Prothero 2017).
Simpson (1945) considered Geolabis as a genus of uncertain position in Insectivora. McKenna (1960b) established the subfamily Geolabidinae as a group of erinacaeid insectivores, which was elevated to family rank by Butler (1972). Van Valen (1967) included Geolabidinae in Adapisoricidae within Erinacaeoidea, while Robinson (1968) included it in Nyctitheriidae, which was explicitly rejected by Novacek (1976). Krishtalka (1975) also supported a close relationship with Nyctitheriidae, while Krishtalka & West (1979) agreed with Novacek (1976) on a closer relationship with a palaeoryctid stock (also see Bloch et al. 2004). Butler (1972) was the first to attribute Geolabididae as distinct family to Lipotyphla-Soricomorpha, which was accepted by most subsequent studies (Schmidt-Kittler 1973, McKenna 1975, Novacek 1976, Sigé 1976, Krishtalka & West 1979, Bown & Schankler 1982, Carroll 1988, McKenna & Bell 1997, Bloch et al. 1998, Lopatin 2004b, Asher et al. 2005, Rose 2006a, Gunnell & Bloch 2008).
Butler (1972, 1988) believed that Geolabididae is most closely related to the West Indian Nesophontidae and Solenodontidae, so that Lopatin (2006) even classified Geolabididae in Nesophontoidea as sister group of the recently extinct Nesophontidae. Novacek (1976) said that “I favor removing the Geolabidinae from the Adapisoricidae and raising the Geolabidinae to familial rank, as Butler has proposed (1972). Butler (1972) has remarked that geolabidines show a number of derived similarities with Solenodon and soricids and the allocation of this group to the Soricoidea seems reasonable.” Of course, all these evolutionary musings have to be considered as obsolete, since nowadays Solenodon is no longer considered to be a close relative of Soricidae.
Lillegraven et al. (1981) provided the arguably “most thorough available analyses of a single insectivoran lineage” (Barnosky 1982) and suggested that Centetodon (previously classified as Nyctitheriidae by Simpson 1945) and Geolabididae is “related more closely to nesophontids, soricids, solenodontids, palaeoryctids (sensu lato), and apternodontids than to other lipotyphlans”. However, from a modern point of view this does not say more than an uncertain position in Lipotyphla or its stem group, because the mentioned soricomorph assemblage is not representing a clade. Gunnell et al. (2008) still concurred with this view, even though Asher et al. (2005) had described new material of Centetodon and found this genus as most closely related to hedgehogs, which resonates with the fact that Geolabidinae was originally established as a subfamily of Erinaceidae. It certainly looks like we can at best suppose that Geolabididae is a possible member of Eulipotyphla with uncertain relationship. No progress in more than 75 years of research.
Heterosoricidae
Simpson (1945), McDowell (1958), and Carroll (1988) listed Heterosorex from the Oligocene and Miocene of Europe and China as genus within Soricidae – Soricinae. While some authors had suggested to include Heterosoricinae as subfamily of Soricidae (Van Valen 1967, Repenning 1967, McKenna & Bell 1997, Storch et al. 1998, Lopatin 2002, 2006, Rose 2006a, Gunnell & Bloch 2008, Gunnell et al. 2008), others rejected this and considered Heterosoricidae as a distinct family (Reumer 1987, 1998, Ziegler 1989, Rzebik-Kowalska 1998, Doby 2015), which is so distantly related to Soricidae that its inclusion would make this family polyphyletic. Reumer (1987) even believed “that Heterosoricidae and Soricidae evolved independently from Eocene Nyctitheriidae, and the more primitive Heterosoricidae deviated much earlier” (Lopatin 2006). However, the cladistic study by Gunnell et al. (2008) again found Heterosoricinae as basal branch of Soricidae.
Leptictida
Leptictida is a diverse order (established by McKenna 1975) of early eutherian mammals that are known from the Late Cretaceous (Gypsonictopidae) and Paleogene (Leptictidae) of North America, Europe, and Central Asia. Some leptictid genera like Leptictidium (Pseudorhyncocyonidae) were small bipedal mammals with a hopping locomotion, unlike earlier speculations that they might have been bipedal runners. Some reached lengths of up to 90 cm and likely had an elongate snout.
Most early works considered Leptictida as primitive placental mammals closely related to hedgehogs, thus as erinaceomorph lipotyphlan insectivores (e.g., Matthew 1903, 1909, Gregory 1910, Jaekel 1911, Simpson 1945). While Matthew (1903) even included leptictids in the living family Erinaceidae, Matthew & Granger (1915) only said that “the position of the typical group of this family is unquestionably in the Insectivora, with affinities nearest to the Erinaceidae”. On the other hand, Butler (1956) considered them as much more primitive than other Erinaceomorpha and therefore proposed to classify them in a separate superfamily Leptictoidea. McDowell (1958) excluded leptictids from Lipotyphla and associated them with tree shrews (Tupaioidea) within Menotyphla, which was concurred by Van Valen (1967) who included Leptictidae in the superfamily Tuapaioidea within insectivoran Proteutheria. Robinson (1966), Lillegraven (1969) and Thenius (1969) classified them in Insectivora. Butler (1972) suggested that Leptictidae were not closely related to any living placental order and assigned them to Proteutheria, which was concurred by various other contemporary studies (e.g., McKenna 1975, Novacek 1975, 1976, 1977, Szalay 1977, Kielan-Jaworowska et al. 1979, Rose 1981, Bown & Schankler 1982). Nevertheless, McKenna (1975) and Szalay (1977) classified Leptictidae as close relatives of rodents and lagomorphs, which was also supported by a more recent cladistic study of morphological data (Asher et al. 2005). Novacek (1986) reviewed all the arguments for the alternative relationships of Leptictidae and considered them as basal stock of Insectivora and closest sister group of Lipotyphla. Carroll (1988) considered Leptictida as eutherian mammals of uncertain position. McKenna & Bell (1997) considered Leptictida as a distinct superorder at the base of Epitheria, which is the hypothetical clade of all placental mammals apart from xenarthrans (also see Springer et al. 2004). Returning to earlier views, Kielan-Jaworowska et al. (2004) and Lopatin (2006) suggested that Leptictida is the sister group of Lipotyphla. Rose (2006) also adopted Novacek’s (1986) proposal that orders Leptictida and Lipotyphla are sister groups within a superorder Insectivora. Meehan & Martin (2010) were likely the last to assign Leptictida to Insectivora, which is an obsolete taxon they used quite anachronistically.
While Novacek (1986) still concluded that “are indeed closely related to lipotyphlan insectivorans”, just a few years later and without much new evidence MacPhee & Novacek (1993) did not longer consider Leptictidae as closely related to lipotyphlans. Agusti & Antón (2002) considered Lepticidae as a group of archaic placental mammals and not closely related to living hedgehogs, shrews, and moles. Multiple modern phylogenetic studies recovered Lepticida outside of Placentalia as a basal grade of eutherians (Ji et al. 2002, Wible et al. 2007, 2009, Goswami et al. 2011, Halliday 2015, Halliday et al. 2015, Manz et al. 2015, Velazco et al. 2022; also see Prothero 2017), while others recovered them as relatives of the crown group placental order in Afrotheria – Macroscelidea (e.g., Zack et al. 2005, Hooker & Russell 2012, O’Leary et al. 2013; see Bechly 2022) or of Macroscelidea+Glires (Hooker 2014).
Welcome to the phylogenetics circus, where anything goes and nothing is certain. And as in other groups like Amphilemuridae, Apatomyidae, and Apternodontidae (see above), the shocking uncertainty about leptictid affinities is by no means based on insufficient fossil evidence, as well-preserved and complete skeletons are known from these extinct mammals.
Micropternodontidae
Micropternodontidae are known from the Early Paleocene to Early Miocene of Central Asia and North America. Matthew (1909) listed Micropternodus among Zalambdodonta. Simpson (1945) considered Micropternodus as a Solenodontidae. McDowell (1958) rejected an affinity with Zalambdodonta in general and with Solenodontidae in particular, and instead transferred micropternodontid genera to Nyctitheriidae. Van Valen (1966) included Micropternodontinae as a subfamily of Palaeoryctidae within his new order Deltatheridia (also see Thenius 1969), an obsolete artificial group that also included the extinct carnivore orders Hyaenodontidae and Oxyaenidae. Van Valen (1967) classified them as separate family close to Palaeoryctidae again within the insectivoran order Deltatheridia. Butler (1972) removed Micropternodontidae from Palaeoryctidae, but McKenna et al. (1984) and Wang & Zhai (1995) still classified this family as a palaeoryctoid within soricomorph insectivores, and Averianov (1994) assigned Micropternodontidae to Palaeoryctoidea in uncertain mammalian order. Ting (1998) listed Micropternodontidae as Insectivora, and Lopatin & Kondrashov (2004), who described the new subfamily Sarcodontinae (separated as family by Missiaen & Smith 2008), only assigned them to lipotyphlan insectivores, without specifying soricomorph affinities.
Schmidt-Kittler (1973) and McKenna (1975) thought that Micropternodontidae are possibly related to Soricomorpha, and Carroll (1988) and McKenna & Bell (1997) even included it in Soricoidea. Some experts agreed and included this family in Soricomorpha (Novacek 1976, Lopatin 2003b, 2004b, 2006, Rose 2006a, Gunnell & Bloch 2008, and Gunnell et al. 2008). Asher et al. (2002) recovered Micropternodus and Erinaceidae as the most basal branches of Lipotyphla, but this result must be considered as dubious and unreliable, because the same study also nested Tenrecidae among eulipotyphlan taxa.
Nyctitheriidae
Nyctitheriidae is an enigmatic family of small, insectivorous mammals from the Paleogene of Eurasia and North America. Just like the relationships of Nyctitheriidae with other mammals, there is little consensus on their internal composition and classification (see Robinson 1968, Sigé 1976, Gunnell et al. 2008, Manz & Bloch 2015).
The oldest fossil record seems to be the nyctitheriine Leptacodon proserpinae from the earliest Paleocene (66.043-63.3 mya) of Purgatory Hill in Montana (Van Valen 1978). Other very old Nyctitheriinae have been described from Palaeocene sediments in Alberta (Scott 2003), that are dated to the Late Torrejonian to middle Tiffanian and thus could date between 61.7-58 mya.
There were two dubious reports of alleged Late Cretaceous records of Nyctitheriidae from the Maastrichtian (70.6-66 mya) of Portugal (Antunes et al. 1986) and from the Oldman Formation (84.9-70.6 mya) in Alberta / Canada (Simpson 1928a). However, both records are just brief mentions in checklists without any description. Therefore, they were arguably ignored as unreliable by all subsequent works on Nyctitheriidae, which unanimously mention a Paleocene origin for this family. Kielan-Jaworowska et al. (2004) and Lopatin (2006) also listed a few alleged Late Cretaceous nyctitheriids of the genus Paranyctoides, but this genus is today considered as an eutherian of uncertain affinity and most likely the sister group to the basal eutherian Zhelestidae (Averianov & Archibald 2013).
Nyctitheriidae was established by Simpson (1928b) as a new family in Insectivora, but McDowell (1958) found that “the family cannot with any assurance be referred to the Lipotyphla” and “must be regarded as a convenient “pigeonhole” for forms incertae sedis”. McKenna (1960a, 1960b) questioned the taxonomic unity of this group and instead classified the various genera in Amphilemuridae and Geolabididae. Van Valen (1967) abandoned the concept of Amphilemuridae and instead classified the genera in Adapisoricidae within the four subfamilies Adapisoricinae, Creotarsinae, Geolabidinae, and Nyctitheriinae within the insectivore suborder Erinaceota. The family Nycitheriidae was restored and redefined by Robinson (1966, 1968) and McKenna (1968).
Jones & Beard (2023) commented that “Nyctitheriidae have previously been considered to belong to such disparate extinct eutherian families as Leptictidae and Adapisoriculidae, and some have even been considered to be early bats. … As a family, Nyctitheriidae has commonly been placed within the order Lipotyphla or Eulipotyphla as a member of the superfamily Soricoidea or the suborder Soricomorpha (e.g., Simpson 1945, Bown and Schankler 1982; Lopatin 2006; Gunnell et al. 2007; Ziegler 2007; Secord 2008), although they have occasionally been considered to be more closely related to the suborder Erinaceomorpha (e.g., Robinson 1968; West 1974). Recent phylogenetic analyses have found Nyctitheriidae to be nested within Eulipotyphla (Manz and Bloch 2015; Manz et al. 2015), although analyses of postcranial morphology have argued for affiliation with the superorder Euarchonta (Hooker 2001, 2014, 2021).”
According to the expert Carly Manz (also see Manz & Bloch 2015), Nyctitheriidae have been alternately hypothesized to be bats or the sister group of bats (Marsh 1872, Gingerich 1987. Jones et al. 2022; also see Matthew 1909, Rose 1981: 39, Hand et al. 1994, Hooker 1996, Manz & Bloch 2015), or early primates or stem Euarchonta (Hooker 2001, 2014, Smith et al. 2010, also see Manz & Bloch 2015), but considered by most workers as a distinct family of Insectivora (Simpson 1928b, Robinson 1966, 1968, Gingerich 1987), especially as Lipotyphla (Rose 1981, Crowell et al. 2024) and most recently as Eulipotyphla (Agusti & Antón 2002, Manz & Bloch 2015, Manz et al. 2015, Das et al. 2022).
While many experts (e.g., Simpson 1928b, 1945, McKenna 1975, Novacek 1976, Bown & Schankler 1982, Carroll 1988, Butler 1988, McKenna & Bell 1997, Rose 2006a, Gunnell & Bloch 2008, Missiaen & Smith 2008, and Rose et al. 2012) considered Ncytitheriidae as soricomorph insectivorans, some others (e.g., McKenna 1968, Robinson 1968, Sigé 1976, Hooker 1986) classified this family in Erinaceomorpha. Wang & Zhai (1995) remarked that there seems to be general agreement that the nyctitheriid Leptacodon is one of the most primitive soricomorphs (McKenna 1968, Robinson 1968, Butler 1972, 1988, Krishtalka 1975, 1976b). Kielan-Jaworowska et al. (2004) and Tabuce et al. (2005) classified Nyctitheriidae as Lipotyphla – Soricomorpha with a question mark. Lopatin (2006) mentioned that “it is usually believed that the closest soricomorph relatives of soricids are the Paleogene Holarctic Nyctitheriidae” and classified Nyctitheriidae together with Plesiosoricidae and Soricidae in a common superfamily Soricoidea within soricomorph Lipotyphla. He recognized five nyctitheriid subfamilies, to which Talpilestinae were added by Tong & Wang (2006). Secord (2008) classified Nyctitheriidae in Soricomorpha, and the cladistic study of Gunnell et al. (2008) indeed recovered Nyctitheriidae as basal branch of Soricoidea within Soricomorpha. Beard & Dawson (2009) considered Nyctitheriidae as soricomorph Lipotyphla.
The extensive cladistic study of Manz et al. (2015) recovered Nyctitheriidae within Eulipotyphla, but only with a very low statistical support. Likewise, Manz & Bloch (2015) discussed the controversial relationships of Nyctitheriidae and only included them with question mark in Eulipotyphla, as close relatives of Adunator and Macrocranion (Amphilemuridae).
Nyctitheriidae as Bats: Based on dental similarities, Marsh (1872) classified Nyctitherium as a bat. Matthew (1909) rejected Marsh’s interpretation of Nyctitherium as a bat and instead considered it as a mole (Talpidae). Gingerich (1987) considered the Late Paleocene Wyonycteris chalix as a bat, but Hand et al. (1994) removed Wyonycteris from bats and placed it close to the genus Placentidens in Nyctitheriidae, because most (but not all) nyctitheriids lack key characters (putative synapomorphies) of early bats (also see Manz & Bloch 2015). Hooker (1996) considered “Nyctitheriidae an unlikely stem group for the order Chiroptera” and agreed with the attribution of Wyonycteris to Nyctitheriidae rather than bats. Jones et al. (2022) re-evaluated the relationships of nyctitheriids based on almost 700 morphological characters and found “Nyctitheriidae to be nonmonophyletic as currently constructed. Our analysis finds several nyctitheres—including Eosoricodon terrigena and “Wyonycteris” microtis—to be most closely related to bats among the included taxa, whereas others are recovered sister to Carnivora or outside of crown Laurasiatheria. No nyctitheres in our analysis grouped with members of Eulipotyphla.”
That is a big oopsie indeed, which not just resuscitates the long dead bat hypothesis, but also overturns most of the results from almost 100 years of research on nyctitheriid affinities with insectivores. Over and over again we find that the whole endeavour to base biological systematics on Darwinian evolution has failed miserably. We should better return to a typological Linnean classification. Actually, when we would calculate a strict consensus tree of all published phylogenetic trees of the past decades of modern cladistic and phylogenomic research, I predict that all that would remain would be the Linnean phyla, classes, orders, and families, while all assumed affinities between them would collapse into an unresolved polytomy that rather resembles the lawn-like pattern suggested by Darwin critics.
Oligoryctidae
McDowell (1958) considered the fossil genera Apternodus and Oligoryctes as closely related but not as closely related to Lipotyphla. Van Valen (1967) classified the genus Oligoryctes in a tenrecid subfamily Apternodontinae (also see Novacek 1976 and McKenna & Bell 1997). Bown & Schankler described the new apternodontid genus Parapternodus and considered Oligoryctes as an apternodontid as well. The family Oligoryctidae was established by Asher et al. (2002) for shrew-sized animals from the Eocene and Oligocene of the American Northwest and possibly Asia. The discovered cranial and dental remains of Oligoryctes allow the reconstruction of the complete skull. As already mentioned above, Asher et al. (2002) and Archer et al. (2010) suggested Oligoryctidae as sister group to Parapternodontidae + Soricidae, which contradicts the classification of Oligoryctinae as a subfamily of Apternodontidae and its relationship with Solenodonta. This was accepted by Rose (2006). However, Gunnell (2008: fig. 7.2) found a clade of Apternodontidae, Parapternodontidae, and Oligoryctidae within Soricoidea. Lopatin & Tesakov (2004) and Lopatin (2003a, 2006) still considered Oligoryctes as a soricomorph Apternodontidae. Gunnell & Bloch (2008) also listed Oligoryctidae as Soricomorpha.
Otlestidae
Otlestidae are only known from the Cretaceous of Russia and Kazakhstan, and possibly India (Khosla et al. 2004). Murtoilestes abramovi even dates to the Early Cretaceous (ca. 130 mya) of Russia (Averianov & Skutschas 2000), which should raise eyebrows concerning any potential affinity of this oldest well-known eutherian with crown group placentals. The genus Otlestes was described by Nessov (1985) and originally considered as a subfamily Otlestinae of Palaeoryctidae. Later, Otlestes was considered as the oldest lipotyphlan (and Soricomorpha) by McKenna & Bell (1997), while some other contemporary studies had attributed Otlestidae to Proteutheria (Kielan-Jaworowska & Dashzeveg 1989, Nessov et al. 1994, and Nessov 1997). Archibald et al. (2001) and Archibald (2003) (implicitly) recovered Otlestes among basal eutherians but found Prokennalestes to be even more basal, thus not more closely related to Otlestes. According to Kielan-Jaworowska et al. (2004) the family Otlestidae also includes Prokennalestes and does not belong to Eulipotyphla but represents basal eutherians, which of course fits much better with the stratigraphic age. Khosla et al. (2004) considered Otlestidae as a boreosphenidan mammalian group, which includes the assumed common stem group of marsupial and placental mammals. Rose (2006) still listed Otlestes as possible Soricomorpha but mentioned that it is “better considered a basal eutherian or possibly a zalambdalestoid (Archibald and Averianov, 2001; Averianov and Archibald, 2005).”
Palaeoryctidae
Palaeoryctidae is an archaic group of insectivorous mammals with zalambdodont dentition that lived in the Late Cretaceous until the Early Eocene of North America.
Simpson (1945) had classified Palaeoryctidae in the insectivoran superfamily Tenrecoidea. McDowell (1958) included Deltatheriidae in Palaeoryctidae and considered them as unrelated to Lipotyphla. Romer (1966) placed Palaeoryctidae in the extinct carnivorous order Creodonta. Van Valen (1966, 1967) classified Palaeoryctidae in an insectivoran order Deltatheridia (also see Lillegraven 1969 and Thenius 1969), while Butler (1972) classified them in a separate order Proteutheria and Van Valen (1978) in Insectivora – Proteutheria. Novacek (1976) still listed Palaeoryctidae in Proteutheria but remarked that “the Palaeoryctidae as presently regarded is probably an unnatural category”. Szalay (1977) include Palaeoryctinae as a subfamily of Leptiptictidae in a new order Leptictimorpha within the cohort Glires, together with living rodents and lagomorphs. McKenna (1969) and Lillegraven (1969) derived carnivorans and ungulates from paleoryctids. McKenna (1975) “regarded palaeoryctines as “ernotheres” (along with elephant shrews and lagomorphs), and classified them in a new ordinal category Kennalestida” (Gingerich 1982). Gingerich (1982) thought that “the phyletic history and systematic relationships of Palaeoryctidae remain somewhat uncertain”, but tentatively classified them as Insectivora. McKenna et al. (1984) proposed that Palaeoryctidae like the Late Cretaceous Batodon are primitive Soricomorpha (also accepted by Carroll 1988), but this genus was classified by other experts in Geolabididae (e.g., Lopatin 2006).
So, this family had been attributed to Insectivora (e.g., Simpson 1945, Sloan & Van Valen 1965, Gingerich 1982, Thewissen & Gingerich 1989), Deltatheridia (Van Valen 1966), Proteutheria (Romer 1966, Butler 1972, Novacek 1976, Kielan-Jaworowska 1981, Rose 1981, Bown & Schankler 1982), or Kennalestida (McKenna 1975). McKenna et al. (1984) even placed Palaeoryctidae within Soricomorpha, but numerous other authors denied a relationship with Lipotyphla (McDowell 1958, Van Valen 1966, 1967, Szalay 1977, Butler 1988, MacPhee & Novacek 1993).
Contrary to all these earlier views, Palaeoryctidae was later often considered as a family in the extinct (mir)order Cimolesta or even in Cimolestidae (e.g., McKenna & Bell 1997, Williamson et al. 2011). This was also supported by Rose (2006a), who attributed Cimolesta to the superorder Ferae, together with living pangolins and carnivores, but commented that the relationship of Palaeoryctidae is uncertain.
Agusti & Antón (2002) considered Palaeoryctidae as a group of archaic placental mammals and not closely related to living hedgehogs, shrews, and moles. Scott et al. (2002) classified Palaeoryctidae as Eutheria of uncertain affinity. Bloch et al. (2004) reviewed the history of classification of this family and concluded that the “systematic position of Palaeoryctidae is uncertain despite decades of study”. This agrees with several other studies that found Cimolesta to belong to a grade of basal eutherians outside of crown group Placentalia (Ji et al. 2002, Wible et al. 2007, 2009, Goswami et al. 2011, Hooker 2014, Halliday 2015, Halliday et al. 2015). Therefore, Palaeoryctidae do not represent a valid Cretaceous record of Eulipotyphla or any other placental insectivores.
It is remarkable that the Late Cretaceous Deltatheridium, which is today considered as oldest marsupial (Rougier et al. 1998, Velazco et al. 2022), was classified by Van Valen (1966) within Palaeoryctidae and used for the naming of his new order Deltatheridia, which also included stem carnivores. All this was suggested by eminent experts based on good morphology and Darwinian evolution but is considered as total bonkers today. This should be kept in mind when grand claims are made that comparative anatomy unequivocally proves evolution. It does no such thing but is always just a vague interpretation of ambiguous data reflecting the spirit of the age in biology. Evolutionary biology rather seems to be ruled by arbitrary “tempora mutantur” than by any progress towards some verisimilitude.
Placentidentinae
The genus Placentidens was described by Russell et al. (1973) from the Early Eocene of France. It was considered by earlier authors as plagiomenid stem Dermoptera within Archonta (Russell et al. 1973, Rose & Simons 1977, Carroll 1988, Ducroq et al. 1992) or as Amphilemuridae within erinaceomorph Lipotyphla (McKenna & Bell 1997). Remember, that these two alternatives are today placed in different cohorts (Euarchontoglires vs Laurasiatheria) of placental mammals, so it is not some minor disagreement. Dawson et al. (1986) rejected a position in Dermoptera. Gingerich (1987) suggested that “Placentidens should be included in Chiroptera rather than Dermoptera.” Smith et al. (2002) again assigned Placentidens to Amphilemuridae within erinaceomorph Lipotyphla. More recently, Beard & Dawson (2009) included an expanded Placentidentinae as subfamily of Nyctitheriidae within soricomorph Eulipotyphla, which was concurred by Manz & Bloch (2015) and more recently by Jones & Beard (2023).
Plesiosoricidae
Plesiosoricidae was a widespread family of insectivores from the Early Eocene to Late Miocene of Asia, Europe, and North America (Ziegler 2009). The generic composition is still matter of some debate (Ziegler 2009). Novacek (1976), Carroll (1988), McKenna & Bell (1997), Lopatin (2006), and Rose (2006) classified Plesisoricidae together with Nyctitheriidae and Soricidae in a superfamily Soricoidea. Just like McKenna (1975), Gunnell & Bloch (2008) listed Plesiosoricidae among Soricomorpha. Gunnell et al. (2008) remarked that “Plesiosorex has been shuttled between the erinaceomorphs and soricomorphs in previous studies, but more recent studies (Wilson, 1960; Butler, 1972) and additional material clearly confirm inclusion in the Soricomorpha.” Their cladistic analysis used Plesiosorex as outgroup to Soricidae, which corresponds to the view of Van Valen (1967) that Soricidae arose from a stock within Plesiosoricidae, which he classified as a distinct family within Soricoidea together with Nesophontidae. Butler (1988) also suggested that living shrews share a common ancestor with Plesiosoricidae. On the other hand, Lopatin (2002) compared the dental morphology and emphasized its “evidence of the essential dissimilarity between the Soricidae and the Plesiosoricidae”, and Lopatin (2006) said that this “shows a considerable gap between the Soricidae and Plesiosoricidae”. Nevertheless, Lopatin (2006), Peigné et al. (2009) and Ziegler (2009) still accepted Plesiosoricidae as family in Lipotyphla – Soricomorpha.
Proscalopidae
Proscalopidae were the earliest known eulipotyphlans with burrowing adaptations, that are mostly known from the Oligocene of North America. Proscalops was therefore already considered as “the oldest true Talpidae” by Matthew (1909). Proscalopids were classified as subfamily of Talpidae by Simpson (1945) and Van Valen (1967). Carroll (1988) included Proscalopidae and Talpidae in Soricoidea. A sister group relationship of Proscalopidae and Talpidae within Soricomorpha – Talpoidea seems to be relatively well established (e.g., McKenna & Bell 1997, Lopatin 2006, Rose 2006a, Sánchez-Villagra et al. 2006, Gunnell & Bloch 2008, and Gunnell et al. 2008). Finally, a taxon without controversy, which proves to be the rare exception from the rule.
Quadratodon
Quadratodon sigei was described by DeBast & Smith (2017) from the Early Paleocene of the Hainin Formation in Belgium, based on a few isolated teeth. It was attributed to Erinaceomorpha with uncertain family affinity. Nothing else has been published on this taxon.
Sarcodontidae
The genus Sarcodon (not to be confused with a fungal genus of the same name) was erected by Matthew & Granger (1925) for a new taxon of small mammal from the latest Paleocene of the Gobi desert in Mongolia. Its systematic position remained debated. The original describers did not assign it to any mammalian order and considered it to be a creodont or a carnivorous marsupial.
Van Valen (1966, 1967) assigned Sarcodon to Palaeoryctidae – Micropternodontinae or Palaeoryctoidea – Micropternodontidae within the order Deltatheridia. Szalay & McKenna (1971) largely agreed but placed it in Paleoryctoidea – Deltatheriidae within the order Insectivora. Rose (1981) disagreed and listed Sarcodon among Proteutheria. McKenna et al. (1984), Carroll (1988), McKenna & Bell (1997), and Rose (2006) again included the sarcodontid genera Sarcodon and Prosarcodon in Micropternodontidae, which they either considered as primitive lipotyphlan Soricomorpha or even classified them in Soricoidea. However, Butler (1988) considered the proposed lipotyphlan affinities as based on non-diagnostic plesiomorphies. Averianov (1994) still classified Sarcodon in Palaeoryctoidea – Micropternodontidae but agreed with Matthew & Granger (1925) that those belong to an uncertain mammalian order.
Wang & Zhai (1995) described a new genus Carnilestes from the Paleocene of China, which they considered as primitive lipotyphlan of uncertain family affinity, but which is today considered as a sarcodontid. The sarcodontid genera Sarcodon and Prosarcodon were classified by Wang & Zhai (1995) as Palaeoryctoidea – Micropternodontidae, similar to the other authors before. The shared loss of M3/m3 of these sarcodontids and Carnilestes was considered by these authors as a convergence, because of alleged derived similarities of Carnilestes with Lipotyphla that are absent in sarcodontids. Wang et al. (1998) listed Sarcodon and Prosarcodon as undetermined family within Insectivora. In the same volume Meng et al. (1998) described a new species of Sarcodon and agreed with this uncertain position.
Lopatin & Kondrashov (2004) established a new subfamily of Micropternodontidae named Sarcodontinae from Early Paleocene to Middle Eocene (61.7-37.3 mya) insectivores of East Asia and France (also see Lopatin 2006). However, this subfamily was transferred as distinct family Sarcodontidae to the mirorder Cimolesta by Missiaen & Smith (2008). The latter mirorder was long attributed to the superorder Ferae, which also includes living pangolins and carnivores, but which is today rather considered as a group of basal eutherians. In short: we have no clue what they really are.
Sespedectidae
Simpson (1945) considered Sespedectes as a possible Leptictidae within Erinaceoidea. The genus Sespedectes and its assumed relatives were classified in the subfamily Creotarsinae of the erinaceoid Adapisoridae by Van Valen (1967). Russell et al. (1975) considered Sespedectes as a possible Dormaaliinae within Adapisoricidae. Novacek (1976) classified Sespedectes in the family Adapisoricidae within Erinaceoidea. Less than ten years later, the same author listed Sespedectes within Dormaaliidae (Novacek et al. 1985, followed by Carroll 1988), and Novacek (1985) formally erected as a new subfamily Sespedectinae within Dormaaliidae for these hedgehog-like insectivores, together with amphilemurines. The family Dormaaliidae was later recognized as a synonym of Amphilemuridae but still classified in Erinaceomorpha (e.g., Lopatin 2006, Rose 2006a). McKenna & Bell (1997) elevated Sespedectidae to family rank within Erinaceomorpha, which was also endorsed by Gunnell & Bloch (2008). A cladistic analysis of Gunnell et al. (2008) indeed recovered Sespedectidae as sister group of Erinaceidae within Erinaceomorpha.
The consensus towards an erinaceomorph relationship is relativized by the similarity to Adapisoricidae, which are no longer considered as Erinaceomorpha and to Amphilemuridae, which are of controversial relationship to Erinaceomorpha.
Soricidae
The earliest uncontroversial fossil record of shrews (Soricidae) is Soricolestes soricavus from the Middle Eocene Khaychin Formation of Mongolia (Lopatin 2002), which was attributed to a separate subfamily Soricolestinae.
The alleged Mesozoic representative Cretasorex was described from the Upper Cretaceous of Uzbekistan by Nessov & Gureev (1981) as the earliest member of the living shrew family Soricidae. Nessov et al. (1994) agreed that it is a Soricidae but suggested that it is most likely a late Cenozoic contaminant, which was also considered by McKenna & Bell (1997). Indeed, it was later recognized by Lopatin & Tesakov (2004) to be a close relative of the living genus Sorex and not older than Late Miocene. To be clear though: there is no primary evidence that Cretasorex is a younger contaminant in Cretaceous sediments. This is simply deduced based on its similarity to recent shrews, which does not fit with the evolutionary narrative if it would be of Cretaceous age. Another case for the common theme that theory trumps data in evolutionary biology.
Vastanidae
The monotypic family Vastanidae was described by Bajpai et al. (2005) for the small fossil mammal Vastania sahnia from the Early Eocene of Western India. The authors attributed this extinct group to erinaceomorph insectivores. Nothing else is known about them.
Zalambdalestidae
Zalambdalestidae is a family of small mammals from the Middle and Late Cretaceous until the Early Paleocene of Central Asia, which had an elongated snout and teeth similar to insectivores but a locomotion more similar to modern lagomorphs. Their skull suggests an adaptation to good olfaction and hearing, correlated with a nocturnal way of life.
Simpson (1945) attributed Zalambdalestidae to Erinaceoidea, together with Leptictidae, Dimylidae and living hedgehogs. In some early works Zalambdalestidae were considered as close relatives of Leptictidae within tree shrew-like Insectivora – Tupaioidea (e.g., McDowell 1958, Van Valen 1967, Thenius 1969).
Because of some similarities with hares and rabbits (Lagomorpha), the Zalambdalestidae have been associated with Glires, Anagalida, or at least Euarchontoglires by several studies (e.g., Van Valen 1964, Szalay & McKenna 1971, McKenna 1975, 1982, 1994, Szalay 1977, Novacek & Wyss 1986, McKenna & Bell 1997, Dashzeveg et al. 1998, Archibald et al. 2001, Meng & Wyss 2001, Fostowicz-Frelik & Kielan-Jaworowska 2002, Averianov & Archibald 2005, Kemp 2005, Janis et al. 2008, Fostowicz-Frelik 2016, 2017, López-Torres & Fostowicz-Frelik 2018; also see Bechly 2023c). However, Van Valen (2002) doubted his earlier belief that zalambdalestids and Glires are related. Meng (2004) found neither evidence for a relationship of zalambdalestids and Glires, nor for any close relatives of Glires in the Cretaceous. Rose (2006b) tentatively still classified Zalambdalestidae in Anagalida with Glires, but mentioned that the phylogenetic position is uncertain and that they could rather be basal eutherians (see below).
Carroll (1988) listed Zalambdalestidae as Eutheria order incertae sedis, but mentioned that they “represent a divergent lineage that shares some similarities with rodents and lagomorphs”. Nessov et al. (1994) attributed Zalambdalestidae to his order Mixotheridia, that he considered as possibly equivalent to Scandentia, as it also included living tree shrews. Archibald et al. (2001) considered Zalambdalestidae as a group of basal gliriforms, which would extend their record back into the Cretaceous about 85-90 million years ago (also see Kemp 2005). This was disputed by Meng & Wyss (2001) because zalambdalestids lack the characteristic derived characters of Glires and only share some (convergent) similarities with lagomorphs but not rodents. Fostowicz-Frelik & Kielan-Jaworowska (2002) also emphasized that the dental similarities do “not necessarily indicate a relationship of Zalambdalestidae to Glires”. Kielan-Jaworowska et al. (2004) considered the affinities as not settled.
Asher et al. (2005) suggested a sister group relationship between Zalambdalestidae and Macroscelidae, but also found a position outside of crown Placentalia in some trees. Rose (2006) listed Zalambdalestidae in Anagalida but mentioned that they “may be basal eutherians”. Other studies found Zalambdalestidae to be stem placentals (Novacek et al. 1997, Ji et al. 2002, some trees in Asher et al. 2005, Wible et al. 2005, 2007, 2009, Goswami et al. 2011, Hooker 2014, Halliday 2015, Halliday et al. 2015, Manz et al. 2015, Velazco et al. 2022). The most powerful evidence against a position in crown group placental mammals, is the retained presence of an epipubic bone (Novacek et al. 1997, Rose 2006a, 2006b), which is absent in all living Placentalia but present in monotremes and marsupials as well as stem placentals such as asioryctids (Novacek et al. 1997). Indeed, in the most recent cladistic analysis of these taxa, Velazco et al. (2022) recently established a clade Tamirtheria as sister group of Placentalia, which among others also includes Zalambdalestidae and Leptictidae, thus returning to the view of the first half of the 20th century.
So, just to recapitulate: Based on the same fossil evidence, distinguished experts considered zalambdalestids to be relatives of hedgehogs, or of tree shrews, or of elephant shrews, or of hares and rabbits, or represent eutherian mammals of uncertain affinity, or represent basal stem eutherians. Evolutionary classification looks like a big guessing game. It is reminding me of an aphorism by Friedrich Nietzsche, who said in response to the crude positivism that dominates modern materialist science that “there are no facts, only interpretations”. This also applies to the alleged fact of evolution, that is exposed as a elusive mirage by the ever shifting interpretations of evolutionary classification and phylogenetic research.
Zhelestidae
Zhelestids are a primitive group of Late Cretaceous to Early Paleocene herbivorous mammals that lived in Central Asia, Europe, and North America. They are mostly known by teeth, while only a single reasonably well-preserved skull of a possible zhelestid has been found. Nessov et al. (1994) included Zhelestidae in his order Mixotheridia, which also included Zalambdalestidae and living tree shrews. McKenna & Bell (1997) classified Zhelestinae in the family Gypsonictopidae of the superorder Leptictida. Ji et al. (2002) suggested a possible relationship of zhelestids with lipotyphlans, but most other experts either considered them as stem ungulates (Nessov et al. 1998, Archibald et al. 2001, Archibald 2003, Kielan-Jaworowska et al. 2004, Kemp 2005) or rather as basal eutherians or stem placentals (Ji et al. 2002, Rose & Archibald 2005, Wible et al. 2007, 2009, Goswami et al. 2011, Archibald & Averianov 2012, Tabuce et al. 2013, Hooker 2014, Halliday et al. 2015, Manz et al. 2015). Indeed, David Archibald changed his mind in a more recent study (Archibald & Averianov 2012) and also came to the conclusion that Zhelestidae groups with stem eutherians rather than crown placentals. Rose (2006) still listed them in Ungulatomorpha, but commented that the monophyly of Ungulatomorpha and Zhelestidae is questionable and “their relationship to ungulates are controversial”. The assumed relationship with ungulates (even-toed and odd-toed ungulates as well as elephants etc.) is obsolete anyway, as ungulates are now considered to be a highly unnatural (polyphyletic) grouping.
Molecular Clocks Conflict with the Fossil Record
The fossil record discussed above clearly contradicts the predictions from molecular clock studies which suggested a much older Cretaceous origin and diversification of Eulipotyphla about a 100-70 million years ago (e.g., Springer 1997, Cooper & Fortey 1998, Kumar & Hedges 1998, Benton 1999, Foote et al. 1999, Penny et al. 1999, Archibald 2003, Douady & Douzery 2003, 2009, Springer et al. 2003, 2018, Roca et al. 2004, Bininda-Emonds et al. 2007, Kitazoe et al. 2007, Prasad 2009, Meredith et al. 2011, Suárez et al. 2011, Foley et al. 2016, 2023, Murphy et al. 2012, Osozawa & Wakabayashi 2023; but see O’Leary et al. 2013, Sato et al. 2016, 2019, Phillips & Fruciano 2018, and Upham et al. 2019 for the opposing view of a post K-Pg diversification of eulipotyphlans).
Actually, a growing number of experts thinks that there simply are no crown group placentals at all known from the Cretaceous fossil record (Novacek et al. 1997, Wible et al. 2005, 2007, 2009; Goswami et al. 2011, Archibald & Averianov 2012, Halliday 2015, Halliday et al. 2015, Velazco et al. 2022). The consistent mismatch between molecular clock datings and empirical data from the fossil record is another striking empirical failure of Darwinian theory that should give evolutionary biologists reason to pause. The fact that it does not, shows how much this discipline is suffering from world view bias and dogmatic blinders to any conflicting evidence.
Convergent Origins of Venom
Last but not least, there is a curious fact about insectivorans that is particularly interesting from an intelligent design theoretical point of view: even though there are only very few venomous mammals (i.e., the platypus, vampire bats, and slow loris), there is growing evidence that the ability for venomous bites originated three or even four times independently among eulipotyphlan insectivores (Turvey 2010, Folinsbee 2013, and Casewell et al. 2019). In my view the common phenomenon of multiple convergences in related taxa (see my article on horizontal tooth replacement Bechly 2022) and/or in the same periods of Earth History and/or the same regions could suggest some non-Darwinian and even non-material cause that could be related to Rupert Sheldrake’s idea of morphic resonance. Definitely a question that would merit further study without bias against such unconventional ideas.
Conclusion: Yet Another Abrupt Appearance
We can conclude that Eulipotyphla appeared abruptly in the Paleocene about 66-61.7 million years ago, not just with a diversity of stem group taxa but already with crown group representatives. This is highly unexpected under neo-Darwinism, as we have already seen in all the examples of the other orders we discussed previously in this article series.
We can also conclude that insectivore systematics and phylogeny are a horrible mess that perfectly illustrates the dubious nature of the whole discipline. The history of insectivore systematics proves that neither morphological nor molecular and genetic similarities correlate with a unique nested hierarchy and thus cannot be considered as lending strong support for Darwinian evolution. The one true tree of life seems to be an elusive myth rather than a scientifically established fact. This does not necessarily imply that common descent is false, but it definitely implies that one of its strongest alleged pieces of evidence does not stand up to scrutiny.
In an upcoming Fossil Friday article, we will continue our review of laurasiatherian orders with the highly diverse order of bats.
References
- Agusti J & Antón M 2002. Mammoths, Sabertooths, and Hominids: 65 Million Years of Mammalian Evolution in Europe. Columbia University Press, New York (NY), 328 pp.
- Antunes MT, Sigogneau-Russell D & Russell DE 1986. Sur quelques dents de Mammifères du Crétacé supérieur de Taveiro, Portugal (Note préliminaire) [On several mammal teeth from the Upper Cretaceous of Taveiro, Portugal (preliminary note)]. Comptes Rendus de l’Académie des Sciences à Paris Série II 303(13), 1247–1250.
- Archer M, Beck R, Gott M, Hand S, Godthelp H & Black K 2010. Australia’s first fossil marsupial mole (Notoryctemorphia) resolves controversies about their evolution and palaeoenvironmental origins. Proceedings of the Royal Society B 278(1711), 1498–1506. DOI: https://doi.org/10.1098/rspb.2010.1943
- Archibald JD 2003. Timing and biogeography of the eutherian radiation: fossils and molecules compared. Molecular Phylogenetics and Evolution 28(2), 350–359. DOI: https://doi.org/10.1016/S1055-7903(03)00034-4
- Archibald JD & Averianov AO 2001. Paranyctoides and allies from the Late Cretaceous of North America and Asia. Acta Palaeontologica Polonica 46(4), 533–551. https://www.app.pan.pl/article/item/app46-533.html
- Archibald JD & Averianov A 2012. Phylogenetic analysis, taxonomic revision, and dental ontogeny of the Cretaceous Zhelestidae (Mammalia: Eutheria). Zoological Journal of the Linnean Society 164(2), 361–426. DOI: https://doi.org/10.1111/j.1096-3642.2011.00771.x
- Archibald JD, Averianov AO & Ekdale EG 2001. Late Cretaceous relatives of rabbits, rodents, and other extant eutherian mammals. Nature 414(6859), 62–65. DOI: https://doi.org/10.1038/35102048
- Arnason U & Janke A 2002. Mitogenomic analyses of eutherian relationships. Cytogenetic and Genome Research 96(1-4), 20–32. DOI: https://doi.org/10.1159/000063023
- Arnason U, Adegoke JA, Bodin K, Born EW, Esa YB, Gullberg A, Nilsson M, Short RV, Xu X & Janke A 2002. Mammalian mitogenomic relationships and the root of the eutherian tree. PNAS 99(12), 8151–8156. DOI: https://doi.org/10.1073/pnas.102164299
- Asher RJ 1999. A morphological basis for assessing the phylogeny of the “Tenrecoidea” (Mammalia, Lipotyphla). Cladistics 15(3), 231–252. DOI: https://doi.org/10.1111/j.1096-0031.1999.tb00266.x
- Asher RJ 2001. Cranial Anatomy in Tenrecid Insectivorans: Character Evolution Across Competing Phylogenies. American Museum Novitates 3352, 1–53. DOI: https:/doi.org/10.1206/0003-0082(2001)352<0001:caitic>2.0.co;2
- Asher RJ & Helgen KM 2010. Nomenclature and placental mammal phylogeny. BMC Evolutionary Biology 10:102, 1–9. DOI: https://doi.org/10.1186/1471-2148-10-102
- Asher RJ & Seiffert ER 2010. Systematics of Endemic African Mammals. Chapter 46, pp. 911–928 in: Werdelin L & Sanders WJ (eds). Cenozoic Mammals of Africa. University of California Press, Berkeley (CA), 1008 pp. DOI: https://doi.org/10.1525/california/9780520257214.003.0046
- Asher RJ, McKenna MC, Emry RJ, Tabrum AR & Kron DG 2002. Morphology and relationships of Apternodus and other extinct, zalambdodont, placental mammals. Bulletin of the American Museum of Natural History 273, 1–117. http://hdl.handle.net/2246/440
- Asher RJ, Novacek MJ & Geisler JH 2003. Relationships of Endemic African Mammals and Their Fossil Relatives Based on Morphological and Molecular Evidence. Journal of Mammalian Evolution 10, 131–194. DOI: https://doi.org/10.1023/A:1025504124129
- Asher RJ, Emry RJ & McKenna MC 2005. New material of Centetodon (Mammalia, Lipotyphla) and the importance of (missing) DNA sequences in systematic paleontology. Journal of Vertebrate Paleontology 25(4), 911–923. DOI: https://doi.org/10.1671/0272-4634(2005)025[0911:nmocml]2.0.CO;2
- Asher RJ, Bennett N & Lehmann T 2009. The new framework for understanding placental mammal evolution. BioEssays 31(8), 853–864. DOI: https://doi.org/10.1002/bies.200900053
- Averianov A 1994. A new species of Sarcodon (Mammalia, Palaeoryctoidea) from the lower Eocene of Kirgizia. Geobios 27(2), 255–258. DOI: https://doi.org/10.1016/S0016-6995(94)80012-X
- Averianov A & Archibald JD 2005. Mammals from the mid-Cretaceous Khodzhakul Formation, Kyzylkum Desert, Uzbekistan. Cretaceous Research 26(4), 593–608. DOI: https://doi.org/10.1016/j.cretres.2005.03.007
- Averianov AO & Archibald JD 2013. New material and reinterpretation of the Late Cretaceous eutherian mammal Paranyctoides from Uzbekistan. Acta Palaeontologica Polonica 58(1), 17–23. DOI: https://doi.org/10.4202/app.2011.0131
- Averianov A & Skutschas P 2000. A eutherian mammal from the Early Cretaceous of Russia and biostratigraphy of the Asian Early Cretaceous vertebrate assemblages. Lethaia 33(4), 330–340. DOI: https://doi.org/10.1080/002411600750053899
- Bajpai S, Kapur VV, Das DP, Tiwari BN, Saravanan N & Sharma R 2005. Early Eocene land mammals from Vastan Lignite Mine, District Surat (Gujarat), Western India. Journal of the Palaeontological Society of India 50(1), 101–113.
- Barnosky AD 1982. [Review] Evolutionary Relationships of Middle Eocene and Younger Species of Centetodon (Mammalia, Insectivora, Geolabididae) with a Description of the Dentition of Ankylodon (Adapisoricidae) by Jasson A. Lillegraven, Malcolm C. McKenna and Leonard Krishtalka, 1981. … . Journal of Vertebrate Paleontology 2(2), 261–267. https://www.jstor.org/stable/4522899
- Beard KC & Dawson MR 2009. Early Wasatchian Mammals of the Red Hot Local Fauna, Uppermost Tuscahoma Formation, Lauderdale County, Mississippi. Annals of Carnegie Museum 78(3), 193–243. DOI: https://doi.org/10.2992/007.078.0301
- Bechly G 2022. Fossil Friday: Fossil Elephant Shrews and the Abrupt Origin of Macroscelidea. Evolution News December 30, 2022. https://evolutionnews.org/2022/12/fossil-friday-fossil-elephant-shrews-and-the-abrupt-origin-of-macroscelidea/
- Bechly G 2023a. Fossil Friday: Golden Moles and the Abrupt Origin of Afrosoricida. Evolution News January 6, 2023. https://evolutionnews.org/2023/01/fossil-friday-fossil-golden-moles-and-the-abrupt-origin-of-afrosoricida/
- Bechly G 2023b. Fossil Friday: The Abrupt Origins of Treeshrews (Scandentia) and Colugos (Dermoptera). Evolution News February 3, 2023. https://evolutionnews.org/2023/02/fossil-friday-the-abrupt-origins-of-treeshrews-scandentia-and-colugos-dermoptera/
- Bechly G 2023c. Fossil Friday: The Abrupt Origins of Lagomorphs and Rodents. Evolution News February 10, 2023. https://evolutionnews.org/2023/02/fossil-friday-the-abrupt-origins-of-lagomorphs-and-rodents/
- Bechly G 2023d. Fossil Friday: The Mess of Arachnid Phylogeny, and Why I’ve Become More Skeptical of Common Descent. Evolution News December 1, 2023. https://evolutionnews.org/2023/12/fossil-friday-the-mess-of-arachnid-phylogeny-and-why-ive-become-more-skeptical-of-common-descent/
- Beck RMD, Bininda-Emonds ORP, Cardillo M, Liu F-GR & Purvis A 2006. A higher level MRP supertree of placental mammals. BMC Evolutionary Biology 6:93, 1–14. DOI: https://doi.org/10.1186/1471-2148-6-93
- Benton MJ 1999. Early origins of modern birds and mammals: molecules vs. morphology. BioEssays 21(12), 1043–1051. DOI: https://doi.org/10.1002/(SICI)1521-1878(199912)22:1<1043::AID-BIES8>3.0.CO;2-B
- Benton MJ, Donoghue PCJ, Asher RA, Friedman M, Near TJ & Vinther J 2015. Constraints on the timescale of animal evolutionary history. Palaeontologia Electronica 18.1.1FC, 1–107. DOI: https://doi.org/10.26879/424 (also see: https://fossilcalibrations.org)
- Biltueva L & Vorobieva N 2012. Chromosome Evolution in Eulipotyphla. Cytogenetic and Genome Research 137(2-4), 154–164. DOI: https://doi.org/10.1159/000339889
- Bininda-Emonds ORP, Cardillo M, Jones KE, MacPhee RDE, Beck RMD, Grenyer R, Price SA, Vos RA, Gittleman JL, Purvis A 2007. The delayed rise of present-day mammals. Nature 446(7135), 507–512. DOI: https://doi.org/10.1038/nature05634
- Bloch JI, Gingerich PD & Rose KD 1998. New species of Batodonoides (Lipotyphla, Geolabididae) from the early Eocene of Wyoming: smallest known mammal? Journal of Mammalogy 79(3), 804–827. DOI: https://doi.org/10.2307/1383090
- Bloch JI, Secord R & Gingerich PD 2004. Systematics and phylogeny of late Paleocene and early Eocene Palaeoryctinae (Mammalia, Insectivora) from the Clarks Fork and Bighorn basins, Wyoming. Contributions from the Museum of Paleontology, The University of Michigan 31(5), 119–154. https://hdl.handle.net/2027.42/48672
- Bown TM & Schankler D 1982. A review of the Proteutheria and Insectivora of the Willwood Formation (Lower Eocene), Bighorn Basin, Wyoming. United States Geological Survey Bulletin 1523, iv+79 pp, 10 pls.
- Brace S, Thomas JA, Dalén L, Burger J, MacPhee RDE, Barnes I & Turvey ST 2016. Evolutionary History of the Nesophontidae, the Last Unplaced Recent Mammal Family. Molecular Biology and Evolution 33(12), 3095–3103. DOI: https://doi.org/10.1093/molbev/msw186
- Brady PL & Springer MS 2021. The effects of fossil taxa, hypothetical predicted ancestors, and a molecular scaffold on pseudoextinction analyses of extant placental orders. PLoS ONE 16(9):e0257338, 1–18. DOI: https://doi.org/10.1371/journal.pone.0257338
- Butler PM 1956. The skull of Ictops and the classification of Insectivora. Proceedings of the Zoological Society of London 126(3), 453–481. DOI: https://doi.org/10.1111/j.1096-3642.1956.tb00449.x
- Butler PM 1972. The problem of insectivore classification. pp. 253–265 in: Joycey KA & Kemp TS (eds). Studies in Vertebrate Evolution. Oliver & Boyd, Edinburgh (UK), ix+284 pp.
- Butler PM 1988. Phylogeny of the Insectivores. pp. 117–141 in: Benton MJ (ed.). The Phylogeny and Classification of the Tetrapods. Volume 2: Mammals. The Systematics Association Special Volume 35B. Clarendon Press, Oxford (UK), x+329 pp.
- Cao Y, Fujiwara M, Nikaido M, Okada N & Hasegawa M 2000. Interordinal relationships and timescale of eutherian evolution as inferred from mitochondrial genome data. Gene 259(1-2), 149–158. DOI: https://doi.org/10.1016/S0378-1119(00)00427-3
- Campbell B 1939. The shoulder anatomy of mole. A study in phylogeny adaptation. American Journal of Anatomy 64(1), 1–39. DOI: https://doi.org/10.1002/aja.1000640102
- Carroll RL 1988. Vertebrate Paleontology and Evolution. WH Freeman & Co, New York (NY), xiv+698 pp.
- Casewell NR, Petras D, Card DC et al. 2019. Solenodon genome reveals convergent evolution of venom in eulipotyphlan mammals. PNAS 116(51), 25745–25755. DOI: https://doi.org/10.1073/pnas.1906117116
- Colbert EH 1969. Evolution of the Vertebrates. 2nd ed. John Wiley & Sons, New York (NY), 535 pp.
- Cooper A & Fortey R 1998. Evolutionary explosions and the phylogenetic fuse. Trends in Ecology & Evolution 13(4), 151–156. DOI: https://doi.org/10.1016/S0169-5347(97)01277-9
- Corneli PS 2002. Complete Mitochondrial Genomes and Eutherian Evolution. Journal of Mammalian Evolution 9(4), 281–305. DOI: https://doi.org/10.1023/A:1023926013667
- Crowell JW, Wible JR & chester SGB 2024. Basicranial evidence suggests picrodontid mammals are not stem primates. Biology Letters 20(1): 20230335, 1–6. DOI: https://doi.org/10.1098/rsbl.2023.0335
- Cuvier G 1798. Tableau élémentaire de l’histoire naturelle des animaux. Baudouin, Paris (F), 770 pp. DOI: https://doi.org/10.5962/bhl.title.11203
- Cuvier G 1817. Le règne animal distribué d’après son organisation : pour servir de base a l’histoire naturelle des animaux et d’introduction a l’anatomie comparée. Déterville, Paris (F), 430 pp. DOI: https://doi.org/10.5962/bhl.title.41460
- Das DP, Carolin N & Bajpai S 2022. A nyctitheriid insectivore (Eulipotyphla, Mammalia) of Asian affinity from the early Eocene of India. Historical Biology 34(7), 1157–1165. DOI: https://doi.org/10.1080/08912963.2021.1966002
- Dashzeveg D, Hartenberger J-L, Martin T & Legendre S 1998. A peculiar minute Glires (Mammalia) from the early Eocene of Mongolia. Bulletin of Carnegie Museum of Natural History 34, 194–209. https://www.researchgate.net/publication/233924881
- Dawson MR & Krishtalka L 1984. Fossil history of the families of recent mammals. pp. 11–57 in: Anderson S & and Knox Jones J (eds). Orders and Families of Recent Mammals of the World. Wiley, New York (NY), xiv+686 pp.
- Dawson M, Hickey LJ, Johnson K & Morrow CJ Jr 1986. Discovery of a dermopteran skull from the Paleogene of Arctic Canada. National Geographic Research 2(1), 112–115.
- De Bast E & Smith T 2017. The oldest Cenozoic mammal fauna of Europe: implication of the Hainin reference fauna for mammalian evolution and dispersals during the Paleocene. Journal of Systematic Palaeontology 15(9), 741–785. DOI: https://doi.org/10.1080/14772019.2016.1237582
- De Bast E, Sigé B & Smith T 2012. Diversity of the Adapisoriculid Mammals from the Early Palaeocene of Hainin, Belgium. Acta Palaeontologica Polonica 57(1), 35-52. DOI: https://doi.org/10.4202/app.2010.0115
- Doby J 2015. A Systematic Review of the Soricimorph Eulipotyphla (Soricidae: Mammalia) from the Gray Fossil Site (Hemphillian), Tennessee. Electronic Theses and Dissertations. Paper 2526, 97 pp. https://dc.etsu.edu/etd/2526
- Douady CJ & Douzery EJ 2003. Molecular estimation of eulipotyphlan divergence times and the evolution of “Insectivora.” Molecular Phylogenetics and Evolution 28(2), 285–296. DOI: https://doi.org/10.1016/s1055-7903(03)00119-2
- Douady CJ & Douzery EJP 2009. Hedgehogs, shrews, moles, and solenodons (Eulipotyphla). pp. 495-498 in: Hedges SB & Kumar S (eds). The Timetree of Life. Oxford University Press, New York (NY), 551 pp. https://timetree.org/public/data/pdf/Douady2009Chap77.pdf
- Douady CJ, Chatelier PI, Madsen O, Jong WW, Catzeflis F, Springer MS & Stanhope MJ 2002. Molecular phylogenetic evidence confirming the Eulipotyphla concept and in support of hedgehogs as the sister group to shrews. Molecular Phylogenetics and Evolution 25(1), 200–209. DOI: https://doi.org/10.1016/S1055-7903(02)00232-4
- Dubey S, Salamin N, Ohdachi SD, Barrière P & Vogel P 2007. Molecular phylogenetics of shrews (Mammalia: Soricidae) reveal timing of transcontinental colonizations. Molecular Phylogenetics and Evolution 44(1), 126–137. DOI: https://doi.org/10.1016/j.ympev.2006.12.002
- Ducrocq S, Buffetaut E, Buffetaut-Tong H, Jaeger J-J, Jongkanjanasoontorn Y & Suteethorn V 1992. First fossil flying lemur: a dermopteran from the Late Eocene of Thailand. Palaeontology 35(2), 373–380. https://www.palass.org/publications/palaeontology-journal/archive/35/2/article_pp373-380
- Dunn RH & Rasmussen DT 2009. Skeletal Morphology of a New Genus of Eocene Insectivore (Mammalia, Erinaceomorpha) from Utah. Journal of Mammalogy 90(2), 321–331. DOI: https://doi.org/10.1644/08-MAMM-A-084.1
- Eberle JJ, Rybczynski N & Greenwood DR 2014. Early Eocene mammals from the Driftwood Creek beds, Driftwood Canyon Provincial Park, northern British Columbia. Journal of Vertebrate Paleontology 34(4), 739–746. DOI: https://doi.org/10.1080/02724634.2014.8381
- Emerson GL, Kilpatrick CW, McNiff BE, Ottenwalder J & Allard MW 1999. Phylogenetic Relationships of the Order Insectivora Based on Complete 12S rRNA Sequences from Mitochondria. Cladistics 15(3), 221–230. DOI: https://doi.org/10.1111/j.1096-0031.1999.tb00265.x
- Esselstyn JA, Oliveros CH, Swanson MT & Faircloth BC 2017. Investigating Difficult Nodes in the Placental Mammal Tree with Expanded Taxon Sampling and Thousands of Ultraconserved Elements. Genome Biology and Evolution 9(9), 2308–2321. DOI: https://doi.org/10.1093/gbe/evx168
- Foley NM, Springer MS & Teeling EC 2016. Mammal madness: Is the mammal tree of life not yet resolved? Philosophical Transactions of the Royal Society B 371(1699):20150140, 1–11. DOI: https://doi.org/10.1098/rstb.2015.0140
- Foley NM, Mason VC; Harris AJ; Bredemeyer KR; Damas J; Lewin HA; Eizirik E; Gatesy J; Karlsson EK; Lindblad-Toh K; Zoonomia Consortium; Springer MS; Murphy WJ; Andrews G; Armstrong JC 2023. A genomic timescale for placental mammal evolution. Science 380(6643): eabl8189. DOI: https://doi.org/10.1126/science.abl8189
- Folinsbee KE 2013. Evolution of venom across extant and extinct eulipotyphlans. Comptes Rendus Palevol 12(7-8), 531–542. DOI: https://doi.org/10.1016/j.crpv.2013.05.004
- Foote M, Hunter JP, Janis CM & Sepkoski JJ Jr 1999. Evolutionary and Preservational Constraints on Origins of Biologic Groups: Divergence Times of Eutherian Mammals. Science 283(5406), 1310–1314. DOI: https://doi.org/10.1126/science.283.5406.1310
- Fostowicz-Frelik Ł 2016. A new zalambdalestid (Eutheria) from the Late Cretaceous of Mongolia and its implications for the origin of Glires. Palaeontologia Polonica 67, 127–136. http://www.palaeontologia.pan.pl/PP67/Fostowicz.pdf
- Fostowicz-Frelik Ł 2017. Convergent and Parallel Evolution in Early Glires (Mammalia). pp. 199–216 in: Pontarotti P (ed.). Evolutionary Biology: Self/Nonself Evolution, Species and Complex Traits Evolution, Methods and Concepts.Springer, Cham (CH), x+396 pp. DOI: https://doi.org/10.1007/978-3-319-61569-1_11
- Fostowicz-Frelik Ł & Kielan-Jaworowska Z 2002. Lower incisor in zalambdalestid mammals (Eutheria) and its phylogenetic implications. Acta Palaeontologica Polonica 47, 177–180. https://www.app.pan.pl/article/item/app47-177.html
- Frost DR, Wozencraft WC & Hoffmann RS 1991. Phylogenetic relationships of hedgehogs and gymnures (Mammalia, Insectivora, Erinaceidae). Smithsonian Contributions to Zoology 518, 1–69. DOI: https://doi.org/10.5479/si.00810282.518
- Gheerbrant E 1988. Afrodon chleuhi nov. gen., nov. sp., “insectivore” (Mammalia, Eutheria) lipotyphlé (?), du Paléocène marocain: données préliminaires. Comptes Rendus de l’Académie des Sciences (Série II) 307, 1303–1309. https://hal-mnhn.archives-ouvertes.fr/mnhn-02264823
- Gheerbrant E 1991. Bustylus (Eutheria, Adapisoriculidae) and the absence of ascertained marsupials in the Palaeocene of Europe. Terra Nova 3(6), 586–592. DOI: https://doi.org/10.1111/j.1365-3121.1991.tb00200.x
- Gheerbrant E 1995. Les mammifères paléocènes du Bassin d’Ouarzazate (Maroc): III. Adapisoriculidae et autres mammifères (Carnivora, ?Creodonta, Condylarthra, ?Ungulata et incertae sedis). Palaeontographica Abt. A 237(1-4), 39–132. DOI: https://doi.org/10.1127/pala/237/1995/39
- Gheerbrant E & Hartenberger JL 1999. Nouveau mammifère insectivore (?Lipotyphla, ?Erinaceomorpha) de l’Eocène inférieur de Chambi (Tunisie). Paläontologische Zeitschrift 73(1/2), 143–156. DOI: https://doi.org/10.1007/BF02987988
- Gheerbrant E & Russell DE 1989. Presence of the genus Afrodon [Mammalia, Lipotyphla (?), Adapisoriculidae] in Europe; new data from the problem of trans-Tethyan relations between Africa and Europe around the K/T boundary. Palaeogeography, Palaeoclimatology, Palaeoecology 76(1-2), 1–15. DOI: https://doi.org/10.1016/0031-0182(89)90099-0
- Gheerbrant E & Russell DE 1991. Bustylus cernaysi nov. gen., nov. sp., new palaeocene Adapisoriculid (Mammalia, Eutheria) from Europe. Géobios 24(4), 467–481. DOI: https://doi.org/10.1016/S0016-6995(06)80247-0
- Gheerbrant E, Sudre J, Sen S, Abrial C & Marandat B 1998. Nouvelles données sur les mammifères du Thanétien et de l’Yprésien du bassin d’Ouarzazate (Maroc) et leur contexte stratigraphique. Palaeovertebrata 27(3-4),155–202. https://palaeovertebrata.com/Articles/sendFile/230/published_article
- Gill TN 1975. Synopsis of Insectivorous Mammals. Bulletin of the United States Geological and Geographical Survey of the Territories (Series 2) No. 2, 91–120. https://catalog.hathitrust.org/Record/100443972
- Gill TN 1884-1885. Insectivora. pp. 134–158 in: Kingsley JS (ed.). The Standard Natural History, Vol. 5: Mammals. Cassino, Boston (MA), viii+535 pp. DOI: https://doi.org/10.5962/bhl.title.12300
- Gingerich PD 1981. Radiation of early Cenozoic Didymoconidae (Condylarthra, Mesonychia) in Asia, with a new genus from the early Eocene of Western North America. Journal of Mammalogy 62(3), 526–538. DOI: https://doi.org/10.2307/1380400
- Gingerich PD 1982. Aaptoryctes (Palaeoryctidae) and Thelysia (Palaeoryctidae?): New Insectivorous Mammals from the Late Paleocene and Early Eocene of Western North America. Contributions from the Museum of Paleontology, University of Michigan 26(3), 37–47. https://hdl.handle.net/2027.42/48511
- Gingerich PD 1983. New Adapisoricidae, Pentacodontidae, and Hyop- sodontidae (Mammalia, Insectivora and Condylarthra) from the late Paleocene of Wyoming and Colorado. Contributions from the Museum of Paleontology, University of Michigan 26(11), 227–255. https://hdl.handle.net/2027.42/48519
- Gingerich PD 1987. Early Eocene bats (Mammalia, Chiroptera) and other vertebrates in freshwater limestones of the Willwood Formation, Clark’s Fork Basin, Wyoming. Contributions from the Museum of Paleontology, The University of Michigan 27(11), 275–320. DOI: https://hdl.handle.net/2027.42/48533
- Gingerich PD & Rose KD 1982. Studies on Paleocene and Early Eocene Apatemyidae (Mammalia, Insectivora): I Dentition of Clarkforkian Labidolemur kayi. II. Labidolemur and Apatemys from the Early Wasatchian of the Clark’s Fork Basin, Wyoming. Contributions from the Museum of Paleontology, The University of Michigan 26(4), 49–69. https://hdl.handle.net/2027.42/48512
- Goswami A, Prasad GVR, Upchurch P, Boyer DM, Seiffert ER, Verma O, Gheerbrant E & Flynn JJ 2011. A radiation of arboreal basal eutherian mammals beginning in the Late Cretaceous of India. PNAS 108(39), 16333–16338. DOI: https://doi.org/10.1073/pnas.1108723108
- Gould GC 1995. Hedgehog phylogeny (Mammalia, Erinaceidae) – the reciprocal illumination of the quick and the dead. American Museum Novitates 3131, 1–45. http://hdl.handle.net/2246/3665
- Gradstein FM, Ogg JG, Schmitz M & Ogg G 2012. The Geologic Time Scale 2012. Elsevier, Amsterdam (NL), 1176 pp.
- Gregory WK 1910. The orders of mammals. Bulletin of the American Museum of Natural History 27, 1–524. DOI: http://hdl.handle.net/2246/313
- Grenyer R & Purvis A 2003. A composite species-level phylogeny of the ‘Insectivora’ (Mammalia: Order Lipotyphla Haeckel, 1866). Journal of Zoology 260(3), 245–257. DOI: https://doi.org/10.1017/S0952836903003716
- Gunnell GF 1989. Evolutionary History of Microsyopoidea (Mammalia, ?Primates) and the Relationship Between Plesiadapiformes and Primates. University of Michigan Papers on Paleontology 27, 1–157. https://lsa.umich.edu/paleontology/publications/papers-on-paleontology/27-The-Evolutionary-History-of-Microsyopoidea-Mammalia-Primates-and-the-Relationship-between-Plesiadapiformes-and-Primates.html
- Gunnell GF & Bloch JI 2008. Part II: Insectivorous mammals. 4 Insectivorous mammals summary. Chapter 4, pp. 49–62 in: Janis CM, Gunnell GF & Uhen MD (eds). Evolution of Tertiary Mammals of North America, Vol. 2: Small Mammals, Xenarthrans, and Marine Mammals. Cambridge University Press, Cambridge (UK), viii+795 pp. DOI: https://doi.org/10.1017/CBO9780511541438.005
- Gunnell GF, Bown T, Hutchison JH & Bloch JI 2008. Part II: Insectivorous mammals. 7 Lipotyphla. Chapter 7, pp. 89–125 in: Janis CM, Gunnell GF & Uhen MD (eds). Evolution of Tertiary Mammals of North America. Volume 2: Small Mammals, Xenarthrans, and Marine Mammals. Cambridge University Press, Cambridge (UK), viii+795 pp pp. DOI: https://doi.org/10.1017/CBO9780511541438.008
- Gutierrez J, Aleix-Mata G, Marchal JA, Arroyo M, Castiglia R & Sánchez A 2020. Molecular Cytogenetic Analysis of Karyotype and Y Chromosome Conservation in Species of the Genus Talpa (Insectivora). Cytogenetic and Genome Research 160(5), 264–271. DOI: https://doi.org/10.1159/000507836
- Haeckel E 1866. Generelle Morphologie der Organismen. Allgemeine Grundzüge der organischen Formen-Wissenschaft, mechanisch begründet durch die von Charles Darwin reformirte Descendenz-Theorie. Georg Reimer, Berlin (DE), Vol. 1 xxxii+574 pp., Vol. 2 clx+462 pp.
- Halliday TJD 2015. The enigmatic evolutionary relationships of Palaeocene mammals and their relevance for the Tertiary radiation of placental mammals. PhD Thesis, UCL, 229 pp. https://discovery.ucl.ac.uk/id/eprint/1469745/
- Halliday TJD, Upchurch P & Goswami A 2015. Resolving the relationships of Paleocene placental mammals. Biological Reviews 92(1), 521–550. DOI: https://doi.org/10.1111/brv.12242
- Hand S, Novacek M, Godthelp H & Archer M 1994. First Eocene bat from Australia. Journal of Vertebrate Paleontology 14(3), 375–381. DOI: https://doi.org/10.1080/02724634.1994.10011565
- He K, Shinohara A, Helgen KM, Springer MS, Jiang X-L & Campbell KL 2017. Talpid Mole Phylogeny Unites Shrew Moles and Illuminates Overlooked Cryptic Species Diversity. Molecular Biology and Evolution 34(1), 78–87. DOI: https://doi.org/10.1093/molbev/msw221
- Honeycutt RL & Adkins RM 1993. Higher Level Systematics of Eutherian Mammals: An Assessment of Molecular Characters and Phylogenetic Hypotheses. Annual Review of Ecology and Systematics 24(1), 279–305. DOI: https://doi.org/10.1146/annurev.es.24.110193.001431
- Holmberg BJ 2022. Ophthalmology of Eulipotyphla: Moles, Shrews, Hedgehogs, and Relatives. pp. 355–366 in: Montiani-Ferreira F, Moore BA & Ben-Shlomo G (eds). Wild and Exotic Animal Ophthalmology. Volume 2: Mammals. Springer, Cham (CH), xx+579 pp. DOI: https://doi.org/10.1007/978-3-030-81273-7_16
- Hooker JJ 1986. Mammals from the Bartonian (middle/late Eocene) of the Hampshire Basin, southern England. Bulletin of the British Museum (Natural History) 39(4), 191–478. https://biostor.org/reference/118600
- Hooker JJ 1996. A Primitive Emballonurid Bat (Chiroptera, Mammalia) from the Earliest Eocene of England. Palaeovertebrata 25(2-4), 287–300. https://palaeovertebrata.com/Articles/sendFile/206/published_article
- Hooker JJ 2001. Tarsals of the extinct insectivoran family Nyctitheriidae (Mammalia): evidence for archontan relationships. Zoological Journal of the Linnean Society 132(4), 501–529. DOI: https://doi.org/10.1111/j.1096-3642.2001.tb02473.x
- Hooker JJ 2014. New postcranial bones of the extinct mammalian family Nyctitheriidae (Paleogene, UK): Primitive euarchontans with scansorial locomotion. Palaeontologia Electronica 17.3.47A, 1–82. DOI: https://doi.org/10.26879/482
- Hooker JJ & Russell DE 2012. Early Palaeogene Louisinidae (Macroscelidea, Mammalia), their relationships and north European diversity. Zoological Journal of the Linnean Society164(4), 856–936. DOI: https://doi.org/10.1111/j.1096-3642.2011.00787.x
- Hu J, Zhang Y & Yu L 2012. Summary of Laurasiatheria (Mammalia) Phylogeny. Zoological Research 33(E5-6), E65–E74. DOI: https://doi.org/10.3724/SP.J.1141.2012.E05-06E65
- Hutterer R 2005a. Order Erinaceomorpha. pp. 212–219 in: Wilson DE & Reeder DM (eds). Mammal Species of the World: A Taxonomic and Geographic Reference. 3rd ed. Johns Hopkins University Press, Baltimore (MD), 2142 pp.
- Hutterer R 2005b. Order Soricomorpha. pp. 220–311 in: Wilson DE & Reeder DM (eds). Mammal Species of the World: A Taxonomic and Geographic Reference. 3rd ed. Johns Hopkins University Press, Baltimore (MD), 2142 pp.
- Huxley TH 1880. On the application of the laws of evolution to the arrangement of the Vertebrata, and more particularly to the Mammalia. Proceedings of the Zoological Society of London 43, 649–662.
- Illiger C 1811. Prodromus systematis mammalium et avium additis terminis zoographicis utriusque classis, eorumque versione germanica. C. Salfeld, Berlin (DE), xviii+301 pp. [In Latin]. DOI: https://doi.org/10.5962/bhl.title.106965
- Jaekel O 1911. Die Wirbeltiere. Eine Übersicht über die Fossilen und Lebenden Formen [The Vertebrates. An Overview of the Fossil and Living Forms]. Gebr. Borntraeger, Berlin (DE), viii-252 pp. [In German]. DOI: https://doi.org/10.5962/bhl.title.119340
- Janis CM, Dawson MR & Flynn LJ 2008. Glires summary. Chapter 16, pp. 263–292 in: Janis CM, Gunnell GF & Uhen MD (eds). Evolution of Tertiary Mammals of North America. Cambridge University Press, Cambridge (UK), viii+795 pp. DOI: https://doi.org/10.1017/CBO9780511541438.017
- Jehle M 2006. Insectivore-like mammals: Tiny teeth and their enigmatic owners. http://www.paleocene-mammals.de/insectivores.htm
- Ji Q, Luo Z-X, Yuan C-X, Wible JR, Zhang J-P & Georgi JA 2002. The earliest known eutherian mammal. Nature 416(6883), 816–822. DOI: https://doi.org/10.1038/416816a
- Jones MF & Beard KC 2023. Nyctitheriidae (Mammalia, ?Eulipotyphla) from the Late Paleocene of Big Multi Quarry, Southern Wyoming, and a Revision of the Subfamily Placentidentinae. Annals of Carnegie Museum 88(2), 115–159. DOI: https://doi.org/10.2992/007.088.0202
- Jones MF, Simmons N & Beard CK 2022. Relationships of nyctitheres (Mammalia, Nyctitheriidae) to bats and other Laurasiatherians. SVP abstracts 2022, 204. https://vertpaleo.org/wp-content/uploads/2022/09/2022-SVP-Program-Abstract-Brochure_Preliminary.pdf
- Kalandadze NN & Rautian SA 1992. Systema mlekopitayushchikh i istorygeskaya zoogeographei [The system of mammals and historical zoogeography]. Sbornik Trudov Zoologicheskogo Muzeya Moskovskogo Goschdarstvennoro Universiteta 29, 44–152.
- Kalthoff DC, Koenigswald Wv & Kurz C 2004. A new specimen of Heterohyus nanus (Apatemyidae, Mammalia) from the Eocene of Messel (Germany) with unusual soft-part preservation. CFS Courier Forschungsinstitut Senckenberg 252, 1–12. https://www.researchgate.net/publication/263714512
- Kemp TS 2005. The Origin and Evolution of Mammals. Oxford University Press, Oxford (UK), 331 pp. [Google Books]
- Khosla A, Prasad GVR, Verma O, Jain AK & Sahni A 2004. Discovery of a micromammal-yielding Deccan intertrappean site near Kisalpuri, Dindori District, Madhya Pradesh. Current Science 87(3), 380–383. https://www.researchgate.net/publication/237488192
- Kielan-Jaworowska Z 1981. Evolution of the therian mammals in the Late Cretaceous of Asia. Part IV. Skull structure in Kennalestes and Asioryctes. Acta Palaeontologica Polonica 42, 25–78. http://www.palaeontologia.pan.pl/Archive/1981-42_25-78_3-19.pdf
- Kielan-Jaworowska Z & Dashzeveg D 1989. Eutherian mammals from the Early Cretaceous of Mongolia. Zoologica Scripta 18(2), 347–355. DOI: https://doi.org/10.1111/j.1463-6409.1989.tb00460.x
- Kielan-Jaworowska Z, Bown TM & Lillegraven JA 1979. Eutheria. pp. 221–259 in: Lillegraven JA, Kielan-Jaworowska Z & Clemens WA (eds). Mesozoic Mammals: The First Two-Thirds of Mammalian History. University of California Press, Berkeley (CA), 311 pp.
- Kielan-Jaworowska Z, Cifelli RL & Luo Z-X 2004. Mammals from the Age of Dinosaurs: Origins, Evolution, and Structure. Columbia University Press, New York (NY), 630 pp. DOI: https://doi.org/10.7312/kiel11918
- Kim NH, Lim SJ, Chae HM & Park YC 2017. Complete mitochondrial genome of the Amur hedgehog Erinaceus amurensis (Erinaceidae) and higher phylogeny of the family Erinaceidae. Genetics and Molecular Research 16(1), 1–8. DOI: https://doi.org/10.4238/gmr16019300
- Kitazoe Y, Kishino H, Waddell PJ, Nakajima N, Okabayashi T, Watabe T & Okuhara Y 2007. Robust Time Estimation Reconciles Views of the Antiquity of Placental Mammals. PLoS ONE 2(4): e384, . DOI: https://doi.org/10.1371/journal.pone.0000384
- Kjer KM, Honeycutt RL 2007. Site specific rates of mitochondrial genomes and the phylogeny of Eutheria. BMC Evolutionary Biology 7(1):8, 1–9. DOI: https://doi.org/10.1186/1471-2148-7-8
- Koenigswald Wv 1987. Apatemyiden-Skelette aus dem Mitteleozän von Messel und ihre paläobiologische Aussage. Carolinea 45, 31–35 [In German]. https://www.zobodat.at/pdf/Carolinea_45_0031-0035.pdf
- Koenigswald Wv 1990. Die Paläobiologie der Apatemyiden (Insectivora s.l.) und die Ausdeutung der Skelettfunde von Heterohyus nanus aus dem Mitteleozän von Messel bei Darmstadt. Palaeontographica A 210(1-3), 41–77. https://www.schweizerbart.de/papers/pala/detail/A210/71360/
- Koenigswald Wv & Storch G 1983. Pholidocercus hassiacus, ein Amphilemuride aus dem Eozan der „Grube Messel“ bei Darmstadt (Mammalia,Lipotyphla). Senckenbergiana Lethaea 64(5-6), 447–495.
- Kretteck A, Gullberg A & Arnason U 1995. Sequence analysis of the complete mitochondrial DNA molecule of the hedgehog, Erinaceus europaeus, and the phylogenetic position of the Lipotyphla. Journal of Molecular Evolution 41(6), 952–957. DOI: https://doi.org/10.1007/BF00173175
- Krishtalka L 1975. Systematics and relationships of early Tertiary Lipotyphla (Mammalia, Insectivora) of North America. PhD Thesis, Texas Tech University, Lubbock (TX)
- Krishtalka L 1976a. Early Tertiary Adapisoricidae and Erinaceidae (Mammalia, Insectivora) of North America. Bulletin of the Carnegie Museum of Natural History 1, 1–40. DOI: https://doi.org/10.5962/p.228580
- Krishtalka L 1976b. North American Nyctitheriidae (Mammalia, Insectivora). Annals of the Carnegie Museum 46:2, 7–28. https://www.biodiversitylibrary.org/item/238343#page/25/mode/1up
- Krishtalka L & West RM 1979. Paleontology and Geology of the Bridger Formation, Southern Green River Basin, Southwestern Wyoming. Part 4. The Geolabididae (Mammalia, Insectivora). Contributions in Biology and Geology, Milwaukee Public Museum 27, 1–10. https://www.mpm.edu/sites/default/files/files%20and%20dox/C%26R/library/bio-geo/%23027%20MPM%20Contributions%20to%20Biology%20and%20Geology%20Number%2027.pdf
- Kumar S & Hedges S 1998. A molecular timescale for vertebrate evolution. Nature 392, 917–920. DOI: https://doi.org/10.1038/31927
- Le Gros Clark WE 1932. The Brain of the Insectivora. Proceedings of the Zoological Society of London 102(4), 975–1013. DOI: https://doi.org/10.1111/j.1096-3642.1932.tb01574.x
- Leche W 1885. Über die Säugethiergattung Galeopithecus. Kungliga Svenska Vetenskaps-Akademiens Handlingar 21, 1–92. (short version as: Leche W 1887. Über die Säugethiergattung Galeopithecus. Zoologische Jahrbücher. Abteilung für Systematik, Geographie und Biologie der Tiere 2, 968–978. https://www.zobodat.at/pdf/Zoologische-Jahrbuecher-Syst_2_0968-0978.pdf)
- Lehmann T 2018. Mit oder ohne Stacheln – die Igel-Verwandten. pp. 235–239 in: Schaal SFK, Smith KT & Habersetzer J (eds). Messel – ein fossiles Tropenökosystem. Senckenberg-Buch 79. Schweizerbart, Stuttgart (DE), xv+355 pp. [In German]. https://www.schweizerbart.de/publications/detail/isbn/9783510614103
- Lillegraven JA 1969. Latest Cretaceous mammals of the upper part of Edmonton Formation of Alberta, Canada, and review of marsupial-placental dichotomy in mammalian evolution. University of Kansas Paleontological Contributions 50, 1–122. http://hdl.handle.net/1808/3825
- Lillegraven JA, McKenna MC & Krishtalka L 1981. Evolutionary relationships of Middle Eocene and younger species of Centetodon (Mammalia, Insectivora, Geolabididae) with a description of the dentition of Ankylodon (Adapisoricidae). University of Wyoming Publications 45, vii+115 pp.
- Linnæi C 1758. Systema Naturæ. 10th edition. Lars Salvius, Stockholm (SE). https://www.biodiversitylibrary.org/item/10277
- Liu F-GR & Miyamoto MM 1999. Phylogenetic assessment of molecular and morphological data for eutherian mammals. Systematic Biology 48(1), 54–64. DOI: https://doi.org/10.1080/106351599260436
- Liu F-GR, Miyamoto MM, Freire NP, Ong PQ, Tennant MR, Young TS & Gugel KE 2001. Molecular and Morphological Supertrees for Eutherian (Placental) Mammals. Science 291(5509), 1786–1789. DOI: https://doi.org/10.1126/science.1056346
- Lopatin AV 2001. The Cranial Structure of Archaeoryctes euryalis sp. nov. (Didymoconidae, Mammalia) from the Paleocene of Mongolia and the Taxonomic Position of the Family. Paleontological Journal 35(3), 320–329 (translated from Paleontologicheskii Zhurnal 3, 97–107). http://repository.geologyscience.ru/bitstream/handle/123456789/25503/Lopat_01.pdf
- Lopatin AV 2002. The earliest shrew (Soricidae, Mammalia) from the Middle Eocene of Mongolia. Paleontological Journal 36(6), 650–659. https://www.researchgate.net/publication/236617908
- Lopatin AV 2003a. A zalambdodont insectivore of the family Apternodontidae (Insectivora, Mammalia) from the Middle Eocene of Mongolia. Paleontological Journal 37(2), 187–195. https://repository.geologyscience.ru/bitstream/handle/123456789/35479/Lopa_03.pdf
- Lopatin AV 2003b. A new species of Ardynictis (Didymoconidae, Mammalia) from the Middle Eocene of Mongolia. Paleontological Journal 37(3), 303–311. https://repository.geologyscience.ru/bitstream/handle/123456789/35477/Lopa_03.pdf
- Lopatin AV 2004a. A New Genus of the Galericinae (Erinaceidae, Insectivora, Mammalia) from the Middle Eocene of Mongolia. Paleontological Journal 38(3), 319–326. https://repository.geologyscience.ru/handle/123456789/39378
- Lopatin AV 2004b. The first find of Geolabididae (Soricomorpha, Mammalia) in Asia (Upper Paleocene of Mongolia). Paleontological Journal 38(6), 672–679. https://repository.geologyscience.ru/handle/123456789/39329
- Lopatin AV 2006. Early Paleogene insectivore mammals of Asia and establishment of the major groups of Insectivora. Paleontological Journal 40(S3), S205–S405. DOI: https://doi.org/10.1134/S0031030106090012
- Lopatin AV & Kondrashov PE 2004. Sarcodontinae, a new subfamily of micropternodontid insectivores from the early Paleocene-middle Eocene of Asia. In: Lucas SG, Zeigler KE & Kondrashov PE (eds). Paleogene Mammals. New Mexico Museum of Natural History and Science Bulletin 26, 177–184. https://www.researchgate.net/publication/236617961
- Lopatin AV & Tesakov AS 2004. The fossil shrew Cretasorex arkhangelskyi Nessov et Gureev, 1981 from Uzbekistan the systematic position among Soricidae, taxonomic status and geological age. Russian Journal of Theriology 3(1), 5–8. https://www.researchgate.net/publication/236617992
- López-Torres S & Fostowicz-Frelik Ł 2018. A new Eocene anagalid (Mammalia: Euarchontoglires) from Mongolia and its implications for the group’s phylogeny and dispersal. Scientific Reports 8:13955, 1–9. DOI: https://doi.org/10.1038/s41598-018-32086-x
- MacPhee RDE, Novacek MJ & Storch G 1988. Basicranial morphology of early Tertiary erinaceomorphs and the origin of primates. American Museum Novitates 2921, 1–42. http://hdl.handle.net/2246/5137
- MacPhee RDE & Novacek MJ 1993. Definition and Relationships of Lipotyphla. pp. 13–31 in: Szalay FS, Novacek MJ & McKenna MC (eds). Mammal Phylogeny. Placentals. Springer, New York (NY), xi+321 pp.
- Madsen O, Scally M, Douady CJ, Kao DJ, DeBry RW, Adkins R, Amrine HM, Stanhope MJ, de Jong WW & Springer MS 2001. Parallel adaptive radiations in two major clades of placental mammals. Nature 409(6820), 610–614. DOI: https/doi.org/10.1038/35054544
- Maier W 1977. Macrocranion tupaiodon Weitzel, 1949, – ein igelartiger Insektivor aus dem Eozän von Messel und seine Beziehungen zum Ursprung der Primaten. Zeitschrift für zoologische Systematik und Evolutionsforschung 15(4), 311–318 [In German]. DOI: https://doi.org/10.1111/j.1439-0469.1977.tb00544.x
- Maier W 1979. Macrocranion tupaiodon, an adapisoricid? Insectivore from the Eocene of ‘Grube Messel’ (Western Germany). Paläontologische Zeitschrift 53(1/2), 38–62. DOI: https://doi.org/10.1007/BF02987787
- Maitre É, Escarguel G & Sigé B 2006. Amphilemuridae (Lipotyphla, Mammalia) éocènes d’Europe occidentale: nouvelles données taxonomiques. Comptes Rendus Palevol 5(6), 813–820. DOI: https://doi.org/10.1016/j.crpv.2006.01.005
- Malia MJ, Adkins RM & Allard MW 2002. Molecular support for Afrotheria and the polyphyly of Lipotyphla based on analyses of the growth hormone receptor gene. Molecular Phylogenetics and Evolution 24(1), 91–101. DOI: https://doi.org/10.1016/s1055-7903(02)00219-1
- Manz CL & Bloch JI 2015. Systematics and Phylogeny of Paleocene-Eocene Nyctitheriidae (Mammalia, Eulipotyphla?) with Description of a new Species from the Late Paleocene of the Clarks Fork Basin, Wyoming, USA. Journal of Mammalian Evolution 22, 307–342. DOI: https://doi.org/10.1007/s10914-014-9284-3
- Manz CL, Chester SGB, Bloch JI, Silcox MT & Sargis EJ 2015. New partial skeletons of Palaeocene Nyctitheriidae and evaluation of proposed euarchontan affinities. Biology Letters 11:20140911, 1–5. DOI: https://doi.org/10.1098/rsbl.2014.0911
- Marsh OC 1872. Preliminary description of new Tertiary mammals. Part I. American Journal of Science Series 3 4(20), 122–128, 202–224. DOI: https://doi.org/10.2475/ajs.s3-4.20.122
- Matthew WD 1903. A fossil hedgehog from the American Oligocene. Bulletin of the American Museum of Natural History 19, 227–229. http://hdl.handle.net/2246/1499
- Matthew WD 1909. The Carnivora and Insectivora of the Bridger Basin, Middle Eocene. Memoirs of the American Museum of Natural History 9(6), 291–576. http://hdl.handle.net/2246/5744
- Matthew WD & Granger W 1915. A Revision of the Lower Eocene Wasatch and Wind River Faunas. Part V. – Insectivora (continued), Glires, Edentata. Bulletin of the American Museum of Natural History 34, 565–657. https://www.biodiversitylibrary.org/bibliography/89493
- Matthew WD & Granger W 1925. Fauna and correlation of the Gashato Formation of Mongolia. American Museum Novitates 189, 1-12.
- Matthew WD, Gregory WK, & Mosenthal JK 1910. Outline classification of the Mammalia, recent and extinct. pp. 511–604 in: Osborn HF (ed.). The Age of Mammals in Europe, Asia and North America. Macmillan, New York (NY).
- McDowell SB 1958. The Greater Antillean insectivores. Bulletin of the American Museum of Natural History 115, 115–214. http://hdl.handle.net/2246/1199
- McKenna MC 1960a. Fossil Mammalia from the early Wasatchian Four Mile fauna, Eocene of northwest Colorado. University of California Publications in Geological Sciences 37(1), 1–130.
- McKenna MC 1960b. The Geolabidinae: a new subfamily of early Cenozoic erinaceoid insectivores. University of California Publications in Geological Sciences 37(2), 131–164.
- McKenna MC 1963. Primitive Paleocene and Eocene Apatemyidae (Mammalia, Insectivora) and the Primate-Insectivore Boundary. American Museum Novitates 2160, 1–39. http://hdl.handle.net/2246/3342
- McKenna MC 1968. Leptacodon, an American Paleocene nyctithere (Mammalia, Insectivora). American Museum Novitates 2317, 1–12. http://hdl.handle.net/2246/2527
- McKenna MC 1969. The origin and early differentiation of therian mammals. Annals of the New York Academy of Sciences 167(1), 217–240. DOI: https://doi.org/10.1111/j.1749-6632.1969.tb20446.x
- McKenna MC 1975. Toward a Phylogenetic Classification of the Mammalia. pp. 21–46 in: Luckett WP & Szalay FS (eds.). Phylogeny of the primates: a multidisciplinary approach. Proceedings of WennerGren Symposium no. 61, Burg Wartenstein, Austria, July 6–14, 1974. Plenum Press, New York (NY). DOI: https://doi.org/10.1007/978-1-4684-2166-8_2
- McKenna MC 1982. Lagomorph interrelationships. Geobios 15(1), 213–223. DOI: https://doi.org/10.1016/S0016-6995(82)80115-0
- McKenna MC 1994. Early relatives of Flopsy, Mopsy, and Cottontail. Natural History 103, 56–58.
- McKenna MC & Bell SK 1997. Classification of Mammals Above the Species Level. Colombia University Press, New York (NY), xii+631 pp. https://books.google.at/books?id=zS7FZkzIw-cC
- McKenna MC, Xue X & Zhou M 1984. Prosarcodon lonanensis, a new Paleocene micropternodontid palaeoryctoid insectivore from Asia. American Museum Novitates 2780, 1–17. http://hdl.handle.net/2246/5265
- Meehan TJ & Martin LD 2010. New leptictids (Mammalia: lnsectivora) from the Early Oligocene of Nebraska, USA. Neues Jahrbuch für Geologie und Paläontologie – Abhandlungen 256(1), 99–107. DOI: https://doi.org/10.1127/0077-7749/2010/0035
- Meng J 2004. Chapter 7: Phylogeny and Divergence of Basal Glires. Bulletin of the American Museum of Natural History 285, 93–109. DOI: https://doi.org/10.1206/0003-0090(2004)285<0093:C>2.0.CO;2
- Meng J & Wyss AR 2001. The morphology of Tribosphenomys (Rodentiaformes, Mammalia): phylogenetic implications for basal Glires. Journal of Mammalian Evolution 8(1), 1–71. DOI: https://doi.org/10.1023/A:1011328616715
- Meng J, Ting S & Schiebout JA 1995. The Cranial Morphology of an Early Eocene Didymoconid (Mammalia, Insectivora). Journal of Vertebrate Paleontology 14(4), 534–551. DOI: https://doi.org/10.1080/02724634.1995.10011576
- Meng J, Zhai R & Wyss AR 1998. The Late Paleocene Bayan Ulan Fauna of Inner Mongolia, China. pp. 148–185 in: Beard KC & Dawson MR (eds). Dawn of the Age of Mammals in Asia. Bulletin of Carnegie Museum of Natural History 34, 1–348. https://www.biodiversitylibrary.org/page/52329896#page/7/mode/1up
- Meredith RW, Janečka JE, Gatesy J et al. 2011. Impacts of the Cretaceous Terrestrial Revolution and KPg Extinction on Mammal Diversification. Science 334(6055), 521–524. DOI: https://doi.org/10.1126/science.1211028
- Missiaen P & Smith T 2008. The Gashatan (late Paleocene) mammal fauna from Subeng, Inner Mongolia. Acta Palaeontologica Polonica 53(3), 357–378. https://www.app.pan.pl/archive/published/app53/app53-357.pdf
- Missiaen P, Solé F, de Bast E, Yang J, Li C-S & Smith T 2013. A new species of Archaeoryctes from the Middle Paleocene of China and the phylogenetic diversification of Didymoconidae. Geologica Belgica 16(4), 245–253. https://popups.uliege.be/1374-8505/index.php?id=4273&lang=en
- Miyamoto M & Goodman M 1986. Biomolecular systematics of eutherian mammals: phylogenetic patterns and classification. Systematic Zoology 35(2), 230–240. https://www.jstor.org/stable/2413433
- Mouchaty SK, Gullberg A, Janke A & Arnason U 2000a. Phylogenetic position of the tenrecs (Mammalia: Tenrecidae) of Madagascar based on the analysis of the complete mitochondrial genome sequence of Echinops telfairi. Zoologica Scripta 29(4), 307–317. DOI: https://doi.org/10.1046/j.1463-6409.2000.00045.x
- Mouchaty SK, Gullberg A, Janke A & Arnason U 2000b. The Phylogenetic Position of the Talpidae Within Eutheria Based on Analysis of Complete Mitochondrial Sequences. Molecular Biology and Evolution 17(1), 60–67. DOI: https://doi.org/10.1093/oxfordjournals.molbev.a026238
- Murphy WJ, Eizirik E, Johnson WE, Zhang YP, Ryder OA & O’Brien SJ 2001a. Molecular phylogenetics and the origins of placental mammals. Nature 409(6820), 614–618. DOI: https://doi.org/10.1038/35054550
- Murphy WJ, Eizirik E, O’Brien SJ, Madsen O, Scally M, Douady CJ, Teeling E, Ryder OA, Stanhope MJ, de Jong WW & Springer MS 2001b. Resolution of the Early Placental Mammal Radiation using Bayesian phylogenetics. Science 294(5550), 2348–2351. DOI: https://doi.org/10.1126/science.1067179
- Murphy WJ, Pringle TH, Crider TA, Springer MS & Miller W 2007. Using genomic data to unravel the root of the placental mammal phylogeny. Genome Research 17(4), 413–421. DOI: https://doi.org/10.1101/gr.5918807
- Murphy WJ, Janecka JE, Stadler T, Eizirik E, Ryder OA, Gatesy J, Meredith RW & Springer MS 2012. Response to Comment on “Impacts of the Cretaceous Terrestrial Revolution and KPg Extinction on Mammal Diversification.” Science 337(6090), 34–34. DOI: https://doi.org/10.1126/science.1220078
- Nessov LA 1985. [New mammals from the Cretaceous of Kyzylkum]. Vestn. Leningr. Univ. 17, 8–18. [In Russian]
- Nessov LA 1997. Cretaceous Non-Marine Vertebrates of Northern Eurasia. University St. Petersburg, St. Petersburg, 218 pp. [In Russian]. (posthumous work edited by Golovneva LB & Averianov AO)
- Nessov LA & Gureev AA 1981. [The finding of the jaw of the earliest shrew from the Upper Cretaceous of the Kyzylkum Desert]. Doklady Akademii Nauk SSSR 257(4), 1002–1004 [In Russian].
- Nessov LA, Sigogneau-Russell D & Russell DE 1994. A survey of Cretaceous tribosphenic mammals from middle Asia (Uzbekistan, Kazakhstan and Tajikistan), of their geological setting, age and faunal environment. Palaeovertebrata 23, 51–92. https://palaeovertebrata.com/Articles/sendFile/263/published_article
- Nessov LA, Archibald JD & Kielan-Jaworowska Z 1998. Ungulate-like mammals from the Late Cretaceous of Uzbekistan and a phylogenetic analysis of Ungulatomorpha. Bulletin of Carnegie Museum of Natural History 34, 40–88. https://www.biodiversitylibrary.org/page/52329896#page/48/mode/1up
- Nikaido M, Kawai K, Cao Y, Harada M, Tomita S, Okada N & Hasegawa M 2001. Maximum likelihood analysis of the complete mitochondrial genomes of eutherians and a reevaluation of the phylogeny of bats and insectivores. Journal of Molecular Evolution 53(4-5), 508–516. DOI: https://doi.org/10.1007/s002390010241
- Nikaido M, Cao Y, Okada N & Hasegawa M 2003a. The phylogenetic relationships of insectivores with special reference to the lesser hedgehog tenrecs as inferred from the complete sequence of their mitochondrial genome. Genes & Genetic Systems 78(1), 107–111. DOI: https://doi.org/10.1266/ggs.78.107
- Nikaido M, Cao Y, Harada M, Okada N & Hasegawa M 2003b. Mitochondrial phylogeny of hedgehogs and monophyly of Eulipotyphla. Molecular Phylogenetics and Evolution 28(2), 276–284. DOI: https://doi.org/10.1016/S1055-7903(03)00120-9
- Novacek MJ 1976. Insectivora and Proteutheria of the later Eocene (Uintan) of San Diego County, California. Contributions in Science (Los Angeles) 283, 1–53. DOI: https://doi.org/10.5962/p.241252
- Novacek MJ 1977. A review of Paleocene and Eocene Leptictidae (Eutheria: Mammalia) from North America. PaleoBios 24, 1–42.
- Novacek MJ 1985. The Sespedectinae, a new subfamily of hedge- hog-like insectivores. American Museum Novitates 2822, 1–24. http://hdl.handle.net/2246/5234
- Novacek M 1986. The Skull of Leptictid Insectivorans and the Higher-Level Classification of Eutherian Mammals. Bulletin of the American Museum of Natural History 183, 1–111. http://hdl.handle.net/2246/1628
- Novacek MJ 1992. Mammalian phylogeny: shaking the tree. Nature 356(6365), 121–125. DOI: https://doi.org/10.1038/356121a0
- Novacek MJ, McKenna MC, Neff NA & Cifelli RL 1983. Evidence from earliest known erinaceomorph basicranium that insectivores and primates are not closely related. Nature 306(5944), 683–684. DOI: https://doi.org/10.1038/306683a0
- Novacek MJ & Wyss AR 1986. Higher-level relationships of recent eutherian orders: morphological evidence. Cladistics 2(4), 257–287. DOI: https://doi.org/10.1111/j.1096-0031.1986.tb00463.x
- Novacek MJ, Bown TM & Schankler D 1985. On the classification of early Tertiary Erinaceomorpha (Insectivora, Mammalia). American Museum Novitates 2813, 1–22. http://hdl.handle.net/2246/5283
- Novacek M, Rougier G, Wible J, McKenna MC, Dashzeveg D & Horovitz I 1997. Epipubic bones in eutherian mammals from the Late Cretaceous of Mongolia. Nature 389(6650), 483–486. DOI: https://doi.org/10.1038/39020
- Nowak RM 1999. Insectivores. pp. 169–229 in: Walker’s Mammals of the World. Vol 1. 6th edn. The Johns Hopkins University Press, Baltimore (MD), ixxx+1936 pp.
- O’Leary MA, Bloch JI, Flynn JJ et al. 2013. The Placental Mammal Ancestor and the Post–K-Pg Radiation of Placentals. Science 339 (6120), 662–667. DOI: https://doi.org/10.1126/science.1229237
- Orihuela León J 2023. Revision of the extinct island-shrews Nesophontes (Mammalia: Eulipotyphla: Nesophontidae) from Cuba. Journal of South American Earth Sciences 130: 104544. DOI: https://doi.org/10.1016/j.jsames.2023.104544 (https://doi.org/10.2139/ssrn.4519913)
- Osborn HF 1910. The Age of Mammals in Europe, Asia, and North America. Macmillan, New York (NY), xvii+635 pp. DOI: https://doi.org/10.5962/bhl.title.39523
- Osozawa S & Wakabayashi J 2023. Geologically calibrated mammalian tree and the corresponding global events, including birth of human. Research Square preprint, 33 pp. DOI: https://doi.org/10.21203/rs.3.rs-523676/v1
- Peigné S, Chaimanee Y, Yamee C, Marandat B, Srisuk P & Jaeger JJ 2009. An astonishing example of convergent evolution toward carnivory: Siamosorex debonisi n. gen., n. sp. (Mammalia, Lipotyphla, Soricomorpha, Plesiosoricidae) from the Latest Oligocene of Thailand. Geodiversitas 31(4), 973–992. DOI: https://doi.org/10.5252/g2009n4a973
- Penny D, Hasegawa M, Waddell PJ & Hendy MD 1999. Mammalian Evolution: Timing and Implications from Using the LogDeterminant Transform for Proteins of Differing Amino Acid Composition. Systematic Biology 48(1), 76–93. DOI: https://doi.org/10.1080/106351599260454
- Peters WCH 1864. Über die Säugethier-Gattung Solenodon. Abhandlungen der Königlichen Akademie der Wissenschaften zu Berlin 1863, 1–22.
- Phillips MJ & Fruciano C 2018. The soft explosive model of placental mammal evolution. BMC Evolutionary Biology 18:104, 1–13. DOI: https://doi.org/10.1186/s12862-018-1218-x
- Piccinini M, Kleinschmidt T, Gorr T, Weber RE, Künzle H & Braunitzer G 1991. Primary structure and oxygen binding properties of the hemoglobin from the lesser hedgehog tenrec (Echinops telfairi, Zalambdodonta): Evidence for phylogenetic isolation. Biological Chemistry Hoppe-Seyler 372, 975–989. DOI: https://doi.org/10.1515/bchm3.1991.372.2.975
- Pickford M, Senut B, Morales J, Mein P & Sánchez IM 2008. Mammals from the Lutetian of Namibia. Memoir of the Geological Survey of Namibia 20, 465–514. https://www.mme.gov.na/files/publications/a93_Memoir%2020_2008_Pickford&Senut_Geology%20and%20Palaeobiology%20of%20the%20Northern%20Sperrgebiet.pdf
- Prasad GVR 2009. Divergence time estimates of mammals from molecular clocks and fossils: Relevance of new fossil finds from India. Journal of Biosciences 34(5), 649–659. DOI: https://doi.org/10.1007/s12038-009-0063-x
- Prasad AB, Allard MW & Green ED 2008. Confirming the Phylogeny of Mammals by Use of Large Comparative Sequence Data Sets. Molecular Biology and Evolution 25(9), 1795–1808. DOI: https://doi.org/10.1093/molbev/msn104
- Prothero DR 2017. Laurasiatheria: Insectivores – Order Eulipotyphla and Other Insectivorous mammals. Chapter 9, pp. 103–111 in: The Princeton Field Guide to Prehistoric Mammals. Princeton University Press, Princeton (NJ), 240 pp. DOI: https://doi.org/10.1515/9781400884452-011
- Repenning CA 1967. Subfamilies and genera of the Soricidae. Geological Survey Professional Paper 565, 1–74. DOI: https://doi.org/10.3133/pp565
- Reumer JWF 1987. Redefinition of the Soricidae and the Heterosoricidae (Insectivora, Mammalia), with the Description of the Crocidosoricinae, a New Subfamily of Soricidae. Revue de Paleobiologie 6(2), 189–192. https://www.researchgate.net/publication/263595563
- Reumer JWF 1998. A classification of fossil and recent shrews. pp. 5–22 in: Wójcik JM & Wolsan M (eds). Evolution of Shrews. Polish Academy of Sciences, Białowieża (PL), 458 pp.
- Robinson P 1966. Fossil Mammalia of the Huerfano Formation, Eocene of Colorado. Bulletin of the Peabody Museum of Natural History 21, 1–85. https://elischolar.library.yale.edu/peabody_museum_natural_history_bulletin/21/
- Robinson P 1968. Nyctitheriidae (Mammalia, Insectivora) from the Bridger Formation of Wyoming, USA. Contributions to Geology University of Wyoming 7(2), 129–138. https://pubs.geoscienceworld.org/uwyo/rmg/article-abstract/7/2/129/88007/Nyctitheriidae-Mammalia-Insectivora-from-the?redirectedFrom=fulltext
- Robinson P & Kron DG 1998. Koniaryctes, a new genus of apternodontid insectivore from lower Eocene rocks of the Powder River basin, Wyoming. Contributions to Geology, University of Wyoming 32(2), 187–190. https://pubs.geoscienceworld.org/uwyo/rmg/article-abstract/32/2/187/87911/Koniaryctes-a-new-genus-of-apternodontid
- Roca AL, Bar-Gal GK, Eizirik E, Helgen KM, Maria R, Springer MS, O’Brien SJ & Murphy WJ 2004. Mesozoic origin for West Indian insectivores. Nature 429(6992), 649–651. DOI: https://doi.org/10.1038/nature02597
- Romer AS 1966. Vertebrate Paleontology. 3rd edition. University of Chicago Press, Chicago (IL), 468 pp.
- Rose KD 2006a. Insectivora. Chapter 9, pp. 138–155 in: The Beginning of the Age of Mammals. John Hopkins University Press, Baltimore (MA), xiv+428 pp. https://books.google.at/books?id=3bs0D5ix4VAC
- Rose KD 2006b. Anagalida: Rodents, Lagomorphs, and Their Relatives. pp. 306–334 in: The Beginning of the Age of Mammals. John Hopkins University Press, Baltimore (MA), xiv+428 pp. https://books.google.at/books?id=3bs0D5ix4VAC
- Rose KD 1981. The Clarkforkian Land-Mammal Age and mammalian faunal composition across the Paleocene-Eocene boundary. University of Michigan Papers on Paleontology 26, 1–197. https://hdl.handle.net/2027.42/48626
- Rose KD & Archibald JD 2005. The Rise of Placental Mammals: Origins and Relationships of the Major Extant Clades. Johns Hopkins University Press, Baltimore (MD), xi+259 pp.
- Rose KD & Simons EL 1977. Dental Function in the Plagiomenidae: Origin and Relationships of the Mammalian Order Dermoptera. Contributions from the Museum of Paleontology, The University of Michigan 24(20), 221–236. https://hdl.handle.net/2027.42/48487
- Rose KD, Chew AE, Dunn RH, Kraus MJ, Fricke HC & Zack SP 2012. Earliest Eocene mammalian fauna from the Paleocene-Eocene Thermal Maximum at Sand Creek Divide, southern Bighorn Basin, Wyoming. University of Michigan Papers on Paleontology 36, ix+122 pp. https://hdl.handle.net/2027.42/89881
- Rougier G, Wible J & Novacek M 1998. Implications of Deltatheridium specimens for early marsupial history. Nature 396(6710), 459–463. DOI: https://doi.org/10.1038/24856
- Roux GH 1947. The cranial development of certain ethiopian “insectivores” and its bearing on the mutual affinities of the group. Acta Zoologica 28(2-3), 165–397. DOI: https://doi.org/10.1111/j.1463-6395.1947.tb00025.x
- Russell DE 1964. Les mammifères paléocènes d’Europe. Mémoires du Muséum national d’Histoire naturelle C 13, 1–324.
- Russell DE, Louis P & Savage DE 1973. Chiroptera and Dermoptera of the French early Eocene. University of California Publications in Geological Sciences 95, 1–57.
- Russell DE, Louis P & Savage DE 1975. Les Adapisoricidae de l’Eocène Inférieur de France: Réévaluation des formes considérées affines. Bulletin de Muséum national d’Histoire naturelle (3) [Sciences de la Terre] 45(327), 129–193. https://www.biodiversitylibrary.org/item/246448
- Russell DE, Godinot M, Louis P & Savage DE 1979. Apatotheria (Mammalia) de l’Eocène inférieur de France et de Belgique. Bulletin du Muséum national d’histoire naturelle C 1(3), 203–243.
- Rzebik-Kowalska B 1998. Fossil history of shrews in Europe. pp. 23–92 in: Wójcik JM & Wolsan M (eds). Evolution of Shrews. Polish Academy of Sciences, Białowieża (PL), 458 pp.
- Saban R 1954. Phylogénie des Insectivores. Bulletin du Muséum national d’histoire naturelle Sér. 2 26, 419–432. https://archive.org/details/biostor-253202
- Saban R 1958. Insectivora. pp. 822–909 in: Piveteau J (ed.). Traité de Paléontologie. Tome 6, Vol. 2: L’Origine des Mammifères. Masson, Paris (F), 962 pp.
- Sánchez-Villagra MR, Horovitz I & Motokawa M 2006. A comprehensive morphological analysis of talpid moles (Mammalia) phylogenetic relationships. Cladistics 22(1), 59–88. DOI: https://doi.org/10.1111/j.1096-0031.2006.00087.x
- Sato JJ, Ohdachi SD, Echenique-Diaz LM, Borroto-Páez R, Begué-Quiala G, Delgado-Labañino JL, Gámez-Díez J, Alvarez-Lemus J, Nguyen ST, Yamaguchi N & Kita M 2016. Molecular phylogenetic analysis of nuclear genes suggests a Cenozoic over-water dispersal origin for the Cuban solenodon. Scientific Reports 6(1). DOI: https://doi.org/10.1038/srep31173
- Sato JJ, Bradford TM, Armstrong KN, Donnellan SC, Echenique-Diaz LM, Begué-Quiala G, Gámez-Díez J, Yamaguchi N, Nguyen ST, Kita M & Ohdachi SD 2019. Post K-Pg diversification of the mammalian order Eulipotyphla as suggested by phylogenomic analyses of ultra-conserved elements. Molecular Phylogenetics and Evolution 141:106605, 1–14. DOI: https://doi.org/10.1016/j.ympev.2019.106605
- Schmidt-Kittler N 1973. Dimyloides-Neufunde aus der ober-Oligozänen Spaltenfüllung “Ehrenstein 4” (Süddeutschland) und die systematische Stellung der Dimyliden (Insectivora, Mammalia). Mitteilungen der Bayerischen Staatsammlung für Paläontologie und historische Geologie 13, 115–139. https://www.zobodat.at/pdf/Mitt-Bayer-Staatsslg-Pal-hist-Geol_13_0115-0139.pdf
- Schwermann AH, He K, Peters BJ, Plogschties T & Sansalone G 2019. Systematics and macroevolution of extant and fossil scalopine moles (Mammalia, Talpidae). Palaeontology 62(4), 661–676. DOI: https://doi.org/10.1111/pala.12422
- Scornavacca C, Belkhir K, Lopez J, Dernat R, Delsuc F, Douzery EJP & Ranwez V 2019. OrthoMaM v10: Scaling-Up Orthologous Coding Sequence and Exon Alignments with More than One Hundred Mammalian Genomes. Molecular Biology and Evolution 36(4), 861–862. DOI: https://doi.org/10.1093/molbev/msz015
- Scott CS 2003. Late Torrejonian (Middle Paleocene) Mammals from South Central Alberta, Canada. Journal of Paleontology 77(4), 745–768. https://www.jstor.org/stable/4094821 DOI: https://doi.org/10.1666/0022-3360(2003)077<0745:LTMPMF>2.0.CO;2
- Scott CS 2006. A new erinaceid (Mammalia, Insectivora) from the Late Paleocene of western Canada. Canadian Journal of Earth Sciences 43(11), 1695–1709. DOI: https://doi.org/10.1139/e06-034
- Scott CS, Fox RC & Youzwyshyn GP 2002. New earliest Tiffanian (late Paleocene) mammals from Cochrane 2, southwestern Alberta, Canada. Acta Palaeontologica Polonica 47(4), 691–704. https://www.app.pan.pl/article/item/app47-691.html
- Secord R 2008. The Tiffanian Land-Mammal Age (middle and late Paleocene) in the northern Bighorn Basiin, Wyoming. University of Michigan Papers in Paleontology 35, 1–192. https://digitalcommons.unl.edu/geosciencefacpub/188/
- Seiffert ER 2010a. Paleogene “Insectivores”. Chapter 16, pp. 253–260 in: Werdelin L & Sanders WJ (eds). Cenozoic Mammals of Africa. University of California Press, Berkeley (CA), 1008 pp. DOI: https://doi.org/10.1525/california/9780520257214.003.0016
- Seiffert ER 2010b. The oldest and youngest records of afrosoricid placentals from the Fayum Depression of northern Egypt. Acta Palaeontologica Polonica 55(4), 599–616. DOI: https://doi.org/10.4202/app.2010.0023
- Seiffert ER & Simons EL 2000. Widanelfarasia, a diminutive placental from the late Eocene of Egypt. PNAS 97(6), 2646–2651. DOI: https://doi.org/10.1073/pnas.040549797
- Seiffert ER, Simons EL, Ryan TM, Bown TM & Attia Y 2007. New remains of Eocene and Oligocene Afrosoricida (Afrotheria) from Egypt, with implications for the origin of afrosoricid zalambdodonty. Journal of Vertebrate Paleontology 27(4), 963–972. DOI: https://doi.org/10.1671/0272-4634(2007)27[963:NROEAO]2.0.CO;2
- Senekowitsch M 2013. Eulipotyphla (Mammalia) aus Cheile Turzii (Rumänien). Spezialanpassungen von Insektenfressern. Diplomarbeit, University Vienna, Vienna (AT), 143 pp. https://utheses.univie.ac.at/detail/24912
- Shoshani J & McKenna MC 1998. Higher Taxonomic Relationships among Extant Mammals Based on Morphology, with Selected Comparisons of Results from Molecular Data. Molecular Phylogenetics & Evolution 9(3), 572–584. DOI: https://doi.org/10.1006/mpev.1998.0520
- Sigé B 1975. Insectivores primitifs de l’Eocène supérieur et Oligocène inférieur d’Europe occidentale. Apatemyidés et Leptictidés. pp. 653–672 in: Problèmes actuels de Paléontologie – Evolution des Vertébrés. Colloques Internationales de CNRS218. CNRS, Paris (F), 675 pp.
- Sigé B 1976. Insectivores primitifs de l’Éocène supérieur et Oligocène inférieur d’Europe occidentale. Mémoires du Muséum national d’Histoire naturelle Ser. C 34, 1–140.
- Silcox MT, Bloch JI, Boyer DM & Houde P 2010. Cranial anatomy of Paleocene and Eocene Labidolemur kayi (Mammalia: Apatotheria), and the relationships of the Apatemyidae to other mammals. Zoological Journal of the Linnean Society 160(4), 773–825. DOI: https://doi.org/10.1111/j.1096-3642.2009.00614.x
- Simpson GG 1928a. A catalogue of the Mesozoic Mammalia in the Geological Department of The British Museum. Oxford University Press, London (UK), 215 pp.
- Simpson GG 1928b. A new mammalian fauna from the Fort Union of southern Montana. American Museum Novitates 297, 1–15. http://hdl.handle.net/2246/3165
- Simpson GG 1931 a. A New Classification of Mammals. Bulletin of the American Museum of Natural History 59, 259–293.
- Simpson GG 1931b. A new insectivore from the Oligocene, Ulan Gochu horizon, of Mongolia. American Museum Novitates 505, 1–22. http://hdl.handle.net/2246/2983
- Simpson GG 1945. The Principles of Classification and a Classification of the Mammals. Bulletin American Museum of Natural History 85, ix+350 pp. http://hdl.handle.net/2246/1104
- Shoshani J & McKenna MC 1998. Higher taxonomic relationships among extant mammals based on morphology, with selected comparisons of results from molecular data. Molecular Phylogenetics and Evolution 9(3), 572–584. DOI: https://doi.org/10.1006/mpev.1998.0520
- Sloan RE & Van Valen L 1965. Cretaceous mammals from Montana. Science 148(3667), 220–227. DOI: https://doi.org/10.1126/science.148.3667.220
- Smith T, Bloch JI, Strait SG & Gingerich PD 2002. New species of Macrocranion (Mammalia, Lipotyphla) from the earliest Eocene of North America and its biogeographic implications. Contributions from the Museum of Paleontology, The University of Michigan 30(14), 373–384. https://hdl.handle.net/2027.42/48665
- Smith T, De Bast E & Sigé B 2010. Euarchontan affinity of Paleocene Afro-European adapisoriculid mammals and their origin in the late Cretaceous Deccan Traps of India. Naturwissenschaften 97, 417–422.DOI: https://doi.org/10.1007/s00114-010-0651-5
- Smith T, De Bast E & Sigé B 2010. Euarchontan affinity of Paleocene Afro-European adapisoriculid mammals and their origin in the late Cretaceous Deccan Traps of India. Naturwissenschaften 97, 417–422. DOI: https://doi.org/10.1007/s00114-010-0651-5
- Song S, Liu L, Edwards SV & Wu S 2012. Resolving conflict in eutherian mammal phylogeny using phylogenomics and the multispecies coalescent model. PNAS 109(37), 14942–14947. DOI: https://doi.org/10.1073/pnas.1211733109
- Springer MS 1997. Molecular Clocks and the Timing of the Placental and Marsupial Radiations in Relation to the Cretaceous–Tertiary Boundary. Journal of Mammalian Evolution 4(4), 285–302. DOI: https://doi.org/10.1023/A:1027378615412
- Springer MS, Cleven GC, Madsen O, de Jong WW, Waddell VG, Amrine HM & Stanhope MJ 1997. Endemic African mammals shake the phylogenetic tree. Nature 388(6637), 61–64. DOI: https://doi.org/10.1038/40386
- Springer MS, Murphy WJ, Eizirik E & O’Brien SJ 2003. Placental mammal diversification and the Cretaceous–Tertiary boundary. PNAS 100(3), 1056–1061. DOI: https://doi.org/10.1073/pnas.0334222100
- Springer MS, Stanhope MJ, Madsen O & de Jong WW 2004. Molecules consolidate the placental mammal tree. Trends in Ecology and Evolution 19(8), 430–438. DOI: https://doi.org/10.1016/j.tree.2004.05.006
- Springer MS, Burk-Herrick A, Meredith R, Eizirik E, Teeling E, O’Brien SJ & Murphy WJ 2007a. The Adequacy of Morphology for Reconstructing the Early History of Placental Mammals. Systematic Biology 56(4), 673-684. https://www.jstor.org/stable/20143073
- Springer MS, Murphy WJ, Eizirik E, Madsen O, Scally M, Douady CJ, Teeling EC, Stanhope MJ, de Jong WW & O’Brien SJ 2007b. A molecular classification for the living orders of placental mammals of the phylogenetic placement of primates. Chapter 1, pp. 1–28 in: Ravosa MJ & Dagosto M (eds). Primate Origins: Adaptations and Evolution. Springer, New York (NY), xxx+829 pp. DOI: https://doi.org/10.1007/978-0-387-33507-0_1
- Springer MS, Emerling CA, Meredith RW, Janečka JE, Eizirik E & Murphy WJ 2017. Waking the undead: Implications of a soft explosive model for the timing of placental mammal diversification. Molecular Phylogenetics and Evolution 106, 86–102. DOI: https://doi.org/10.1016/j.ympev.2016.09.017
- Springer MS, Murphy WJ & Roca AL 2018. Appropriate fossil calibrations and tree constraints uphold the Mesozoic divergence of solenodons from other extant mammals. Molecular Phylogenetics and Evolution 121, 158–165. DOI: https://doi.org/10.1016/j.ympev.2018.01.007
- Stanhope MJ, Waddell VG, Madsen O, de Jong W, Hedges SB, Cleven GC, Kao D & Springer MS 1998a. Molecular evidence for multiple origins of Insectivora and for a new order of endemic African insectivore mammals. PNAS 95(17), 9967–9972. DOI: https://doi.org/10.1073/pnas.95.17.9967
- Stanhope MJ, Madsen O, Waddell VG, Cleven GC, de Jong WW & Springer MS 1998b. Highly congruent molecular support for a diverse superordinal clade of endemic African mammals. Molecular Phylogenetics and Evolution 9(3), 501–508. DOI: https://doi.org/10.1006/mpev.1998.0517
- Storch G 1993. Morphologie und Paläobiologie von Macrocranion tenerum, einem Erinaceomorphen aus dem Mittel-Eozän von Messel bei Darmstadt. Senckenbergiana lethaea 73, 61–81.
- Storch G 1996. Paleobiology of Messel Erinaceomorphs. Palaeovertebrata 25(2-4), 215–224. https://palaeovertebrata.com/Articles/sendFile/196/published_article
- Storch G 2008. Skeletal remains of a diminutive primate from the Paleocene of Germany. Naturwissenschaften 95, 927–930. DOI: https://doi.org/10.1007/s00114-008-0401-0
- Storch G, Qiu Z & Zazhigin VS 1998. Fossil history of shrews in Asia. pp. 93–120 in: Wójcik JM & Wolsan M (eds). Evolution of Shrews. Polish Academy of Sciences, Białowieża (PL), 458 pp.
- Suárez R, Fernández-Aburto P, Manger PR & Mpodozis J 2011. Deterioration of the Gαo Vomeronasal Pathway in Sexually Dimorphic Mammals. PLoS ONE 6(10):e26436, 1–6. DOI: https://doi.org/10.1371/journal.pone.0026436
- Symonds MRE 2005. Phylogeny and life histories of the “Insectivora”: controversies and consequences. Biological Reviews 80(1), 93–128. DOI: https://doi.org/10.1017/s1464793104006566
- Szalay FS 1968a. The beginnings of primates. Evolution 22(1), 19–36. DOI: https://doi.org/10.1111/j.1558-5646.1968.tb03445.x
- Szalay FS 1968b. Origins of the Apatemyidae (Mammalia, Insectivora). American Museum Novitates 2352, 1–11.
- Szalay FS 1969. Mixodectidae, Microsyopidae and the insectivore–primate transition. Bulletin of the American Museum of Natural History 140:4, 193–330. http://hdl.handle.net/2246/1130
- Szalay FS 1975. Phylogeny of Primate Higher Taxa: The Basicranial Evidence. pp. 91–125 in: Luckett PW & Szalay FS (eds). Phylogeny of the Primates, a Multidisciplinary Approach. Springer, New York (NY), xiv+483 pp. DOI: https://doi.org/10.1007/978-1-4684-2166-8_5
- Szalay FS 1977. Phylogenetic relationships and a classification of the eutherian Mammalia. pp. 315–374 in: Hecht MK, Goody PC & Hecht BM (eds). Major Patterns in Vertebrate Evolution. Springer, New York (NY), ix+908 pp. DOI: https://doi.org/10.1007/978-1-4684-8851-7_12
- Szalay FS & McKenna MC 1971. Beginning of the age of mammals in Asia: The late Paleocene Gashato fauna, Mongolia. Bulletin of the American Museum of Natural History 144:4, 269–318. http://hdl.handle.net/2246/1085
- Tabuce R, Adnet S, Cappetta H, Noubhani AM & Quillevere F 2005. Aznag (bassin d’Ouarzazate, Maroc), nouvelle localite a selaciens et mammiferes de l’Eocene moyen (Lutetien) d’Afrique. Bulletin de la societe Geologique du France 176(4), 381–400. DOI: https://doi.org/10.2113/176.4.381
- Tabuce R, Tortosa T, Vianey-Liaud M, Garcia G, Lebrun R, Godefroit P, Dutour Y, Berton S, Valentin X & Cheylan G 2013. New eutherian mammals from the Late Cretaceous of Aix-en-Provence Basin, south-eastern France. Zoological Journal of the Linnean Society 169(3), 653–672. DOI: https://doi.org/10.1111/zoj.12074
- Thenius E 1969 (reprint 2019). Phylogenie der Mammalia. Walter de Gruyter, Berlin (DE), 722 pp. [Google Books]
- Thewissen JGM & Gingerich PD 1989. Skull and endocranial cast of Eoryctes melanus, a new palaeoryctid (Mammalia: Insectivora) from the early Eocene of western North America. Journal of Vertebrate Paleontology 9(4), 459–470. DOI: https://doi.org/10.1080/02724634.1989.10011778
- Ting S 1998. Paleocene and early Eocene Land Mammal Ages of Asia. pp. 124–147 in: Beard KC & Dawson MR (eds). Dawn of the Age of Mammals in Asia. Bulletin of Carnegie Museum of Natural History 34, 1–348. https://www.biodiversitylibrary.org/page/52329896#page/7/mode/1up
- Tong Y & Wang J 1993. A New Soricomorph (Mammalia, Insectivora) from the Early Eocene of Wutu Basin, Shandong China. Vertebrata PalAsiatica 31(1), 19–32 [In Chinese and English]. http://www.vertpala.ac.cn/EN/Y1993/V31/I01/19
- Tong Y & Wang J 2006. Fossil Mammals from the Early Eocene Wutu Formation of Shandong Province. Palaeontologica Sinica NS C 192(28), 1–195.
- Turvey ST 2010. The Evolution of Non-Homologous Venom Delivery Systems in West Indian Insectivores? Journal of Vertebrate Paleontology 30(4), 1294–1299. https://www.jstor.org/stable/40864404
- Upham NS, Esselstyn JA & Jetz W 2019. Inferring the mammal tree: Species-level sets of phylogenies for questions in ecology, evolution, and conservation. PLoS Biology 17(12):e3000494, 1–44. DOI: https://doi.org/10.1371/journal.pbio.3000494
- Van Valen L 1964. A Possible Origin for rabbits. Evolution 18(3), 484–491. DOI: https://doi.org/10.1111/j.1558-5646.1964.tb01624.x
- Van Valen L 1966. Deltatheridia, a new order of mammals. Bulletin of the American Museum of Natural History 132, 1–126. http://hdl.handle.net/2246/1126
- Van Valen L 1967. New Paleocene insectivores and insectivore classification. Bulletin of the American Museum of Natural History 135(5), 217–284. http://hdl.handle.net/2246/358
- Van Valen L 1978. The beginning of the Age of Mammals. Evolutionary Theory 4, 45–80. https://www.mn.uio.no/cees/english/services/van-valen/evolutionary-theory/volume-4/vol-4-no-2-pages-45-80-l-van-valen-the-beginning-of-the-age-of-mammals.pdf
- Van Valen L 2002. How did rodents and lagomorphs (Mammalia) originate? Evolutionary Theory 12(5), 101–128. https://www.mn.uio.no/cees/english/services/van-valen/evolutionary-theory/volume-12/vol-12-no-5-pages-101-128-l-van-valen-how-did-rodents-and-lagomorphs-mammalia-originate.pdf
- Velazco PM, Buczek AJ, Hoffman E, Hoffman DK, O’Leary MA & Novacek MJ 2022. Combined data analysis of fossil and living mammals: a Paleogene sister taxon of Placentalia and the antiquity of Marsupialia. Cladistics 38(3), 359–373. DOI: https://doi.org/10.1111/cla.12499
- Vianey-Liaud M, Comte B, Marandat B, Peigné S, Rage J-C & Sudre J 2014. A new early late Oligocene (MP 26) continental vertebrate fauna from Saint-Privat-des-Vieux (Alès Basin, Gard, Southern France). Geodiversitas 36(4), 565–622. DOI: https://doi.org/10.5252/g2014n4a4
- Wagner JA 1855. Die Säugethiere in Abbildungen nach der Natur mit Beschreibungen. Weigel, Leipzig (DE), xxvi+810 pp. https://www.biodiversitylibrary.org/item/98461#page/5/mode/1up
- Waddell PJ, Okada N & Hasegawa M 1999. Towards Resolving the Interordinal Relationships of Placental Mammals. Systematic Biology 48(1), 1–5. DOI: https://doi.org/10.1093/sysbio/48.1.1
- Waddell PJ, Kishino H & Ota R 2001. A Phylogenetic Foundation for Comparative Mammalian Genomics. Genome Informatics 12, 141–154. https://pubmed.ncbi.nlm.nih.gov/11791233/
- Wang X & Zhai R 1995. Carnilestes, a New Primitive Lipotyphlan (Insectivora: Mammalia) from the Early and Middle Paleocene, Nanxiong Basin, China. Journal of Vertebrate Paleontology 15(1), 131–145. http://www.jstor.org/stable/4523612
- Wang Y, Hu Y, Chow M & Li C 1998. Chinese Palaeocene Mammal Faunas and Their Correlation. pp. 89–123 in: Beard KC & Dawson MR (eds). Dawn of the Age of Mammals in Asia. Bulletin of Carnegie Museum of Natural History 34, 1–348. https://www.biodiversitylibrary.org/page/52329896#page/7/mode/1up
- Whidden HP & Asher RJ 2001. The Origin of the Greater Antillean Insectivorans. Chapter 15, pp. 237–252 in: Woods CA & Sergile FE (eds). Biogeography of the West Indies: Patterns and Perspectives. CRC Press, Boca Raton (FL), 608 pp. https://www.taylorfrancis.com/chapters/edit/10.1201/9781420039481-15/origin-greater-antillean-insectivorans-howard-whidden-robert-asher
- Wible JR, Rougier GW & Novacek MJ 2005. Anatomical evidence for superordinal/ordinal Eutherian taxa in the Cretaceous. Chapter 3, pp. 15–36 in: Rose KD & Archibald JD (eds). The Rise of Placental Mammals: Origins and Relationships of the Major Extant Clades. Johns Hopkins University Press, Baltimore (MD), 280 pp. https://books.google.at/books?id=DhchVG_rbQ8C.
- Wible JR, Rougier GW, Novacek MJ & Asher RJ 2007. Cretaceous eutherians and Laurasian origin for placental mammals near the K/T boundary. Nature 447(7147), 1003–1006. DOI: https://doi.org/10.1038/nature05854
- Wible JR, Rougier GW, Novacek MJ & Asher RJ 2009. The eutherian mammal Maelestes gobiensis from the Late Cretaceous of Mongolia and the phylogeny of Cretaceous Eutheria. Bulletin of the American Museum of Natural History 327, 1–123. DOI: https://doi.org/10.1206/623.1
- Williamson TE, Weil A & Standhardt B 2011. Cimolestids (Mammalia) from the early Paleocene (Puercan) of New Mexico. Journal of Vertebrate Paleontology 31(1), 162–180. DOI: https://doi.org/10.1080/02724634.2011.539649
- Wilson DE & Reeder DM (eds) 2005. Mammal Species of the World. A Taxonomic and Geographic Reference. 3rd ed. Johns Hopkins University Press, Baltimore (MD), 2142 pp. http://www.departments.bucknell.edu/biology/resources/msw3/ [first edition 1993]
- Woodburne MO 2004. Late Cretaceous and Cenozoic Mammals of North America: Biostratigraphy and Geochronology. Columbia University Press, New York (NY), ix+391 pp. DOI: https://doi.org/10.7312/wood13040
- Wyss AR 1987. Notes on Prototheria, Insectivora, and Thomas Huxley’s Contribution to Mammalian Systematics. Journal of Mammology 68(1), 135–138. DOI: https://doi.org/10.2307/1381057
- Zachos FE 2020. Mammalian Phylogenetics: A Short Overview of Recent Advances. pp. 1–18 in: Hackländer K & Zachos F (eds). Mammals of Europe – Past, Present, and Future. Handbook of the Mammals of Europe. Springer, Cham (CH), 130 pp. DOI: https://doi.org/10.1007/978-3-319-65038-8_6-1
- Zack SP, Penkrot TA, Bloch JI & Rose KD 2005. Affinities of ‘Hyopsodontids’ to elephant shrews and a Holarctic origin of Afrotheria. Nature 434(7032), 497–501. DOI: https://doi.org/10.1038/nature03351
- Ziegler R 1989. Heterosoricidae und Soricidae (Insectivora, Mammalia) aus dem Oberoligozän und Untermiozän Süddeutschlands. Stuttgarter Beiträge zur Naturkunde B 154, 1–73.
- Ziegler R 2009. Plesiosoricids from early Oligocene fissure fillings in South Germany, with remarks on plesiosoricid phylogeny. Acta Palaeontologica Polonica 54(3), 365–371. DOI: https://doi.org/10.4202/app.2008.0061
