 Life Sciences
Life Sciences
Evolutionary “Gems” or “Narrative Gloss”?
| Links to our 9-Part Series Responding to Nature‘s Evolution Evangelism Packet: • Part 1: Evaluating Nature’s 2009 “15 Evolutionary Gems” Darwin-Evangelism Kit |
In the previous four responses to Nature‘s evolution-evangelism packet, we saw that at least 9 of their 15 “evolutionary gems” showed mere small-scale examples evolutionary change. Despite promises of explaining how “microevolution meets macroevolution” and suggestions that it’s “a fact, in the same way that the Earth orbits the Sun is a fact” that “all life evolved by natural selection,” instead we were treated to discussions of how small coloration spots change in guppies and fruit flies, or how lizards, birds, and fish undergo small-scale changes in body size. While these are undoubtedly legitimate examples of evolution, such slight modifications cannot, as the packet put it, be “extrapolated over long periods of time[to] result in wholesale changes of form and function.” But with some of the “gems” cited by Nature, it’s arguable that they don’t even have anything to do with evolution at all.
In 2005, U.S. National Academy of Sciences member Phil Skell wrote in The Scientist that in his experience, most research — even in biology — does not require evolution. According to Skell, evolution rarely formed the basis of research:
I also examined the outstanding biodiscoveries of the past century: the discovery of the double helix; the characterization of the ribosome; the mapping of genomes; research on medications and drug reactions; improvements in food production and sanitation; the development of new surgeries; and others. I even queried biologists working in areas where one would expect the Darwinian paradigm to have most benefited research, such as the emergence of resistance to antibiotics and pesticides. Here, as elsewhere, I found that Darwin’s theory had provided no discernible guidance, but was brought in, after the breakthroughs, as an interesting narrative gloss.
Phil Skell, “Why Do We Invoke Darwin?,” The Scientist, 2005, Vol. 19(16):10 (2005) (emphasis added).
This seems to be the case for at least two of the evolutionary “gems” in Nature‘s evolution-evangelism packet. As Skell says, evolution is added in later as a “narrative gloss.”
In Praise of Teeth
One “gem from the fossil record” cited by Nature was “The evolutionary history of teeth.” Nature offered only two short paragraphs on this study:
One motivation in the study of development is the discovery of mechanisms that guide evolutionary change. Kathryn Kavanagh at the University of Helsinki and her colleagues investigated just this by looking at the mechanisms behind the relative size and number of molar teeth in mice. The research, published in 2007, uncovered the pattern of gene expression that governs the development of teeth — molars emerge from the front backwards, with each tooth smaller than the last.
The beauty of the study lies in its application. Their model predicts the dentition patterns found in mouse-like rodent species with various diets, providing an example of ecologically driven evolution along a developmentally favored trajectory. In general, the work shows how the pattern of gene expression can be modified during evolution to produce adaptive changes in natural systems.
This is indeed a fascinating study about the mechanisms controlling molar development in mice, but what does it have to do with natural selection? The authors compared relative molar sizes in 29 species of murine rodents and found that the relative sizes fit the patterns predicted by the developmental mechanisms they uncovered in their research. Here’s where the evolutionary interpretation comes in: what’s really a mere comparison of relative tooth sizes in existing murine rodent species is now termed “macroevolutonary data.” At base, they’ve found that all murine rodents follow similar developmental patterns. They term it “macroevolution.”
Perhaps evolution did do all of this. But is this “macroevolutionary data” or is it a study of mere comparative tooth morphology? All this really does is identify molecular mechanisms that explain tooth development in a variety of rodent species.
And if evolution did cause these changes, all we’re talking about is small-scale changes in tooth-sizes within highly similar species of rodents. But no one doubts that mammalian teeth are readily susceptible to evolutionary change. In fact, tooth morphology is so plastic in mammals that many evolutionary systematists find teeth to be nearly useless when studying evolutionary history and constructing supposed phylogenetic trees. As one study in PNAS wrote, “tooth morphology is prone to homoplasy and is therefore a poor guide to low-level phylogenetic relationships.” (Sally Gibbs, Mark Collard, and Bernard Wood, “Soft-tissue characters in higher primate phylogenetics,” Proceedings of the U.S. National Academy of Sciences, Vol. 97:11130-11132 (September 26, 2000).) Likewise another paper reported: “Given the robustness of the molecular phylogenies, these results indicate that little confidence can be placed in phylogenies generated solely from higher primate craniodental evidence.” (Mark Collard and Bernard Wood, “How reliable are human phylogenetic hypotheses?,” Proceedings of the U.S. National Academy of Sciences, Vol. 97: 5003-5006 (April 25, 2000).)
In any case, what this paper terms “macroevolutionary data” is merely small-scale differences in tooth morphology observed by comparing various rodent species. The idea that this provides “macroevolutionary data” is just an interpretative gloss added after the fact.
(See Kathryn D. Kavanagh, Alistair R. Evans, & Jukka Jernvall, “Predicting evolutionary patterns of mammalian teeth from development,” Nature, Vol. 449:427-433 (September 27, 2007).)
Double-Jawed Moray Eels: Very Cool Structures, but What’s Evolution Got to Do With It?
In another gem, Nature‘s evolution-evangelism packet highlights a fascinating feature of certain moray eels which allows its pharyngeal jaws to “move forwards into the mouth cavity, trapping the prey and dragging it backwards” into the gut where digestion can begin. The packet calls this “evolution’s breathtaking solution” to allow moray eels to feed. But the paper is little more than a description of the fascinating feature, with a note that it has striking convergent similarities to the mechanisms that allow snakes to swallow large prey: “The independent evolution of ratcheting mechanisms enables both morays and snakes to maintain a constant grip on their prey.” The last paragraph of the paper states:
The evolution of the newly discovered function and design of this widespread jaw system adds to our general understanding of how innovations arise and how they correlate with a particular body plan. Our discovery demonstrates that striking functional novelties can arise with only subtle modifications in existing systems, and offers new insights into the functional morphology of a successful radiation of predatory fish, the moray eels.
Rita S. Mehta & Peter C. Wainwright, “Raptorial jaws in the throat help moray eels swallow large prey,” Nature 449:79-82 (September 6, 2007).
Huh? The short 3 sentences in the quotes above, which are crammed at the very end of this paper, entail literally everything that this paper says about “evolution.” Apart the fact that moray eel jaws would require much more than mere “subtle modifications” in existing systems to generate this “striking functional novelt[y],” this paper’s bland and vague reference to “evolution” at the end does not offer anything close to an evolutionary explanation. Evolution has been added in only as a narrative gloss.
Where’s the Evolution?
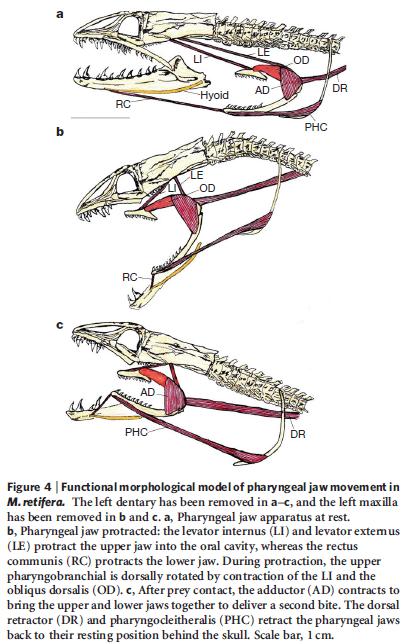
Adapted by permission from Macmillan Publishers Ltd: Nature, Figure 4, Rita S. Mehta & Peter C. Wainwright, “Raptorial jaws in the throat help moray eels swallow large prey,” Nature 449:79-82 (September 6, 2007). Copyright 2007.

