 Evolution
Evolution
Namacalathus, Alleged Ediacaran “Animal,” Fails to Refute Abrupt Cambrian Explosion

Editor’s note: See also, “Namacalathus, an Ediacaran Lophophorate Animal?“
Yesterday, in my series about alleged Ediacaran animals postulated as precursors of the Cambrian explosion, I began to consider Namacalathus, a problematic fossil organism from the Ediacaran Small Shelly Fauna. I referred readers to a sensational study by Zhuravlev et al. (2015) in the Proceedings of the Royal Society and to a range of accompanying media coverage. I asked whether the attribution of Namacalathus to lophophorate animals really is well justified and undisputed. Or is it just another piece of overhyped soft science?
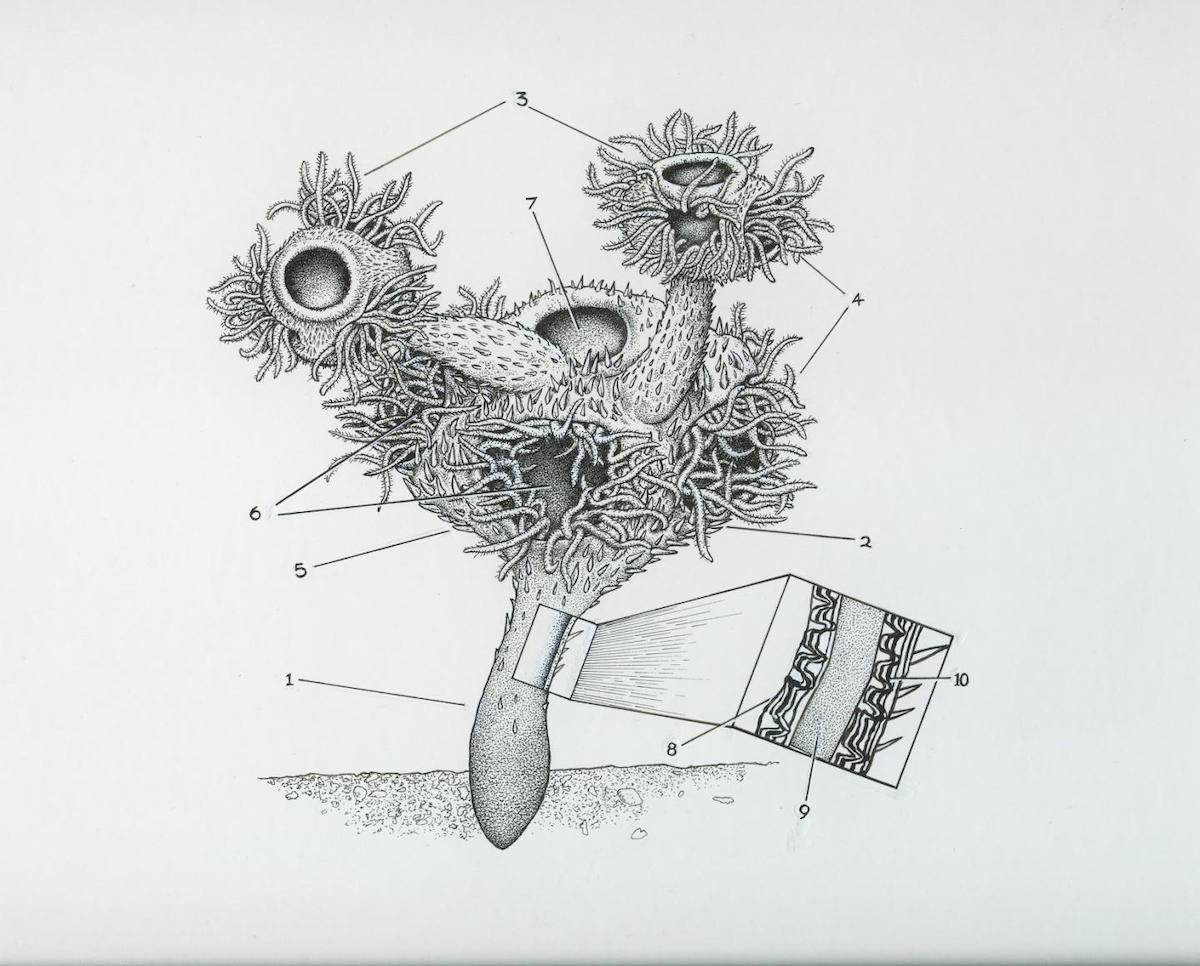
Doubts first arose because Namacalathus is very dissimilar to any early lophotrochozoans (Zhuravlev et al. 2012), and because of its hexaradial symmetry (Warren et al. 2017), as all modern lophophorates have a bilateral symmetry. The authors were quite aware of this problem and therefore postulated that maybe only the skeleton of Namacalathus had a radial symmetry, but that its soft-part anatomy was bilaterally symmetrical. Their only argument for this extraordinary claim was that Namacalathus shows an asexual growth with bilateral budding. However, the authors failed to show if such bilateral budding is really absent in all cnidarians and present in all bilaterians with budding growth.
This might be the case, but it would have to be properly documented for this argument to have any merit. As Cunningham et al. (2017) critically remarked:
This latter interpretation is intriguing, but it is difficult to reconcile with the typically hexaradial symmetry of the cup, which is arguably more consistent with a cnidarian affinity. It is difficult to constrain the affinity of Namacalathus with confidence.
Another problem is that small individuals are randomly attached to larger individuals and thus do not show a bilateral budding pattern. The authors subsequently had to take refuge in another ad hoc hypothesis that those specimens are not due to budding but are just small individuals growing on abandoned skeletons of a larger specimen (Penny et al. 2017). This would mean that the assumed planktonic larvae of Namacalathus did recognize and preferably settle on older skeletons of the same genus, as if intentionally mimicking budding growth. The much more parsimonious hypothesis is that Namacalathus did not grow by obligatory bilateral budding, just like most sessile cnidarians with clonal growth.
Not Convinced? Neither Am I
The only other evidence for the proposed lophophorate affinity is based on the supposedly accretionary growth skeletal wall composed of a foliated external outer layer with robust external wall spines, an internal (middle) organic-rich layer, and an inner foliated layer with columnar microlamellar inflections, which is similar to that in some brachiopod and bryozoan skeletons (Zhuravlev et al. 2012, 2015). However, there is a fatal problem with this argument: this similarity simply cannot be based on homology, because brachiopod and bryozoan skeletons are independently derived (Taylor et al. 2010, Murdock et al. 2020), actually even independently derived twice within Bryozoa, and thus do not belong to the common lophophorate ground plan. Even worse, it is controversial if Lophophorata is a monophyletic group at all, as most modern phylogenomic studies did not consider Bryozoa (= Ectoprocta) as closest relative of brachiopods and phoronids, and rather unite Ectoprocta with Entoprocta and the enigmatic phylum Cycliophora in a clade Polyzoa (e.g., Nielsen 2012, Kocot 2016, Kocot et al. 2017), or even have Bryozoa as basal sister group to all other lophotrochozoans.
Therefore, Landing et al. (2018) thoroughly rebutted the attribution of Namacalathus to Lophophorata and commented:
Zhuravlev et al.’s (2015) use of skeletal histologic features to compare Namacalathus to two lophophorate groups is selective in not noting the variety of histologies in calcareous brachiopods and bryozoan. This comparative approach and conclusion that Namacalathus is a lophophorate assumes that conch ultrastructure is plesiomorphic in these groups although bryozoan biomineralization is apomorphic and arose independently twice from ctenostome-grade taxa. Similarly, features such as pseudopores are limited to strophomenides and acanthostyles to Palaeozoic stenolaematan bryozoans and not characteristic of all calcareous brachiopods and bryozoans.
In the most recent paper by Murdock et al. (2020) the authors agreed in their study of early biomineralization that the affinities of Namacalathus remain equivocal. Obviously unaware of the simultaneous work of Zhuravlev et al. (2015), Butler (2015) discussed Namacalathus but did not include it as putative lophophorate in his revision of early fossil lophophorates. Also, the constantly updated Paleobiology Database lists Namacalathus hermanastes not as a lophophorate but still as a cnidarian.
Other Potential Relatives
Are there any other potential relatives of Namacalathus? Indeed, there are. There is an iconic Cambrian tulip organism called Siphusauctum from the famous Burgess Shale (O’Brien & Caron 2012) and the Middle Cambrian of Utah (Kimmig et al. 2017), which was a sessile filter-feeder with hexaradial symmetry and a stalked cup with apical hole and aboral ring of six openings. Sounds familiar? Together with other problematic Cambrian beasts like Dinomischus, Siphusauctum has recently been recognized as stem-group ctenophores close to Scleroctenophora, a basal clade of sclerotized Cambrian comb jellies (Zhao et al. 2019). A close relationship of Siphusauctum with Namacalathus was also proposed (Zhao et al. 2019, Giribet & Edgecombe 2020: 32) and looks like a more reasonable hypothesis than a lophophorate affinity.
Thus, Namacalathus could be a coelenterate-grade organism after all, as was always thought until the mentioned work of Zhuravlev et al. (2015), who did not even compare the ultastructure of the skeleton with that of sclerotized Cambrian ctenophorans. A problem for this hypothesis is that Siphusauctum seems to be entirely non-mineralized (O’Brien & Caron 2012), and Scleroctenophora had a non-calcified organic skeleton.
So Is It an Animal?
As in all the other cases we looked into with my previous articles in this series, the actual evidence does not support an uncontroversial identification of Namacalathus as an Ediacaran bilaterian animal. Is it an animal (e.g., a ctenophoran) at all? Maybe yes, maybe no. It could be anything, from a coelenterate-grade or sponge-grade organism to even a protist or an alga. Until better evidence becomes available it must be considered as just another problematic Ediacaran organism. Does it refute the abruptness of the Cambrian explosion, with its sudden appearance of at least 17 bilaterian animal phyla and their complex body plans, as was boldly claimed by Penny (2015)? Not even close!
References:
- Amthor JE, Grotzinger JP, Schröder S, Bowring SA, Ramezani J, Martin MW, Matter A 2003. Extinction of Cloudina and Namacalathus at the Precambrian-Cambrian boundary in Oman. Geology 31(5), 431–434. DOI: 10.1130/0091-7613(2003)031<0431:EOCANA>2.0.CO;2.
- Anonymous 2015. Missing Precambrian Animals — Namacalathus? The Palaeobabbler Nov. 7, 2015.
- Antcliffe JB, Callow RHT, Brasier MD 2014. Giving the early fossil record of sponges a squeeze. Biological Reviews 89(4), 972–1004. DOI: 10.1111/brv.12090.
- Bauwens J 2015. Reinterpretation of the Ediacaran Namacalathus as a Lophophorate Animal. Sciency Thoughts Nov. 11, 2015.
- Bechly G 2020a. Did Cloudinids Have the Guts to be Worms. Evolution News Jan. 17, 2020.
- Bechly G 2020b. The Myth of Precambrian Sponges. Evolution News May 12, 2020.
- Bengtson S 2004. Early skeletal fossils. In: Lipps JH, Waggoner BM (eds). Neoproterozoic-Cambrian Biological Revolutions. The Paleontological Society Papers 10, 67–78. DOI: 10.1017/S1089332600002345.
- Brasier MD, Antcliffe JB, Callow RHT 2011. Evolutionary trends in remarkable fossil preservation across the Ediacaran–Cambrian transition and the impact of metazoan mixing. In: Allison PA, Bottjer DJ (eds.). Taphonomy: Process and Bias Through Time. Topics in Geobiology, vol. 32, 519–567. Springer, Dordrecht. DOI: 10.1007/978-90-481-8643-3_15.
- Butler, A. D. 2015. Decoding the fossil record of early lophophorates. Systematics and phylogeny of problematic Cambrian Lophotrochozoa. Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1284, 65 pp. Uppsala: Acta Universitatis Upsaliensis. ISBN: 978-91-554-9327-1. DOI: 10.13140/RG.2.1.1188.1044.
- Cunningham JA, Liu AG, Bengtson S, Donoghue PCJ 2017. The origin of animals: Can molecular clocks and the fossil record be reconciled? BioEssays 39(1), 1–12. DOI: 10.1002/bies.201600120.
- Giribet G, Edgecombe GD 2020. The Invertebrate Tree of Life. Princeton University Press, 608 pp. [Google Books]
- Grant SWF 1990. Shell structure and distribution of Cloudina, a potential index fossil for the terminal Proterozoic. American Journal of Science 290−A. 261–294. PMID: 11538690.
- Grazhdankin DV, Kontorovich AE, Kontorovich VA, Saraev SV, Filippov YF, Efimov AS, Karlova GA, Kochnev BB, Nagovitsin KE, Terleev AA, Fedyanin GO 2015. Vendian of the Fore-Yenisei sedimentary basin (southeastern West Siberia). Russian Geology and Geophysics 56(4), 560–572. DOI: 10.1016/j.rgg.2015.03.008.
- Grotzinger JP, Watters WA, Knoll AH 2000. Calcified metazoans in thrombolite-stromatolite reefs of the terminal Proterozoic Nama Group, Namibia. Paleobiology 26(3), 334–359. DOI: 10.1666/0094-8373(2000)026<0334:CMITSR>2.0.CO;2.
- Grotzinger JP, Adams EW, Schröder S 2005. Microbial–metazoan reefs of the terminal Proterozoic Nama Group (c. 550–543 Ma), Namibia. Geological Magazine 142(5), 499–517. DOI: 10.1017/S0016756805000907.
- Hofmann HJ, Mountjoy EW 2001. Namacalathus–Cloudina assemblage in Neoproterozoic Miette Group (Byng Formation), British Columbia: Canada’s oldest shelly fossils. Geology 29(12), 1091–1094. DOI: 10.1130/0091-7613(2001)029<1091:NCAINM>2.0.CO;2.
- Joel L 2015. Small ‘goblet’ fossil found to be big piece of animal evolutionary history. Washington Post Nov. 21, 2015.
- Kimmig J, Strotz LC, Liebermann BS 2017. The stalked filter feeder Siphusauctum lloydguntheri n. sp. from the middle Cambrian (Series 3, Stage 5) Spence Shale of Utah: its biological affinities and taphonomy. Journal of Paleontology 91(5), 902–910. DOI: 10.1017/jpa.2017.57.
- Kocot KM 2016. On 20 years of Lophotrochozoa. Organisms Diversity & Evolution 16, 329–343. DOI: 10.1007/s13127-015-0261-3.
- Kocot KM, Struck TH, Merkel J, Waits DS, Todt C, Brannock PM, Weese DA, Cannon JT, Moroz LL, Lieb B, Halanych KM 2017. Phylogenomics of Lophotrochozoa with Consideration of Systematic Error. Systematic Biology 66(2), 256–282. DOI: 10.1093/sysbio/syw079.
- Kontorovich AE, Varlamov AI, Grazhdankin DV, Karlova GA, Klets AG, Kontorovich VA, Saraev SV, Terleev AA, Belyaev SY, Varaksina IV, Efimov AS, Kochnev BB, Nagovitsin KE, Postnikov AA, Filippov YF 2008. A section of Vendian in the east of West Siberian Plate (based on data from the Borehole Vostok 3). Russian Geology and Geophysics 49(12), 932–939. DOI: 10.1016/j.rgg.2008.06.012.
- Kontorovich AE, Sokolov BS, Kontorovich VA, Varlamov AI, Grazhdankin DV, Efimov AS, Klets AG, Saraev SV, Terleev AA, Belyaev SY, Varaksina IV, Karlova GA, Kochnev BB, Nagavitsin KE, Postnikov AA, Filippov YF 2009. The first section of vendian deposits in the basement complex of the West Siberian petroleum megabasin (resulting from the drilling of the Vostok-3 parametric borehole in the Eastern Tomsk region). Doklady Earth Sciences 425, 219–222. DOI: 10.1134/S1028334X09020093.
- Kouchinsky A, Bengtson S, Runnegar B, Skovsted C 2012. Chronology of early Cambrian biomineralization. Geological Magazine 149(2), 221–251. DOI: 10.1017/S0016756811000720.
- Landing E, Antcliffe JB, Geyer G, Kouchinsky A, Bowser SS, Andreas A 2018. Early evolution of colonial animals (Ediacaran Evolutionary Radiation–Cambrian Evolutionary Radiation–Great Ordovician Biodiversification Interval). Earth-Science Reviews 178, 105–135. DOI: 10.1016/j.earscirev.2018.01.013.
- Murdock DJE 2020. The ‘biomineralization toolkit’ and the origin of animal skeletons. Biological Reviews. DOI: 10.1111/brv.12614.
- National Geographic Kids 2015. Have scientists discovered the earliest ever animal?
- Nielsen C 2012. Animal Evolution: Interrelationships of the Living Phyla. Oxford University Press, 402 pp. [Google Books]
- O’Brien LJ, Caron J-B 2012. A new stalked filter-feeder from the Middle Cambrian Burgess Shale, British Columbia, Canada. PLoS ONE 7(1), e29233, 1–21. DOI: 10.1371/journal.pone.0029233.
- Penny A 2015. A complex animal from the beginning of animal life. Serious Science Dec. 8, 2015.
- Penny AM, Wood RA, Zhuravlev AY, Curtis A, Bowyer F, Tostevin R 2017. Intraspecific variation in an Ediacaran skeletal metazoan: Namacalathus from the Nama Group, Namibia. Geobiology 15(1), 81–93. DOI: 10.1111/gbi.12205.
- Porter SM 2007. Seawater Chemistry and Early Carbonate Biomineralization. Science 316(5829), 1302. DOI: 10.1126/science.1137284.
- Pruss SB, Blättler CL, Macdonald FA, Higgins JA 2018. Calcium isotope evidence that the earliest metazoan biomineralizers formed aragonite shells. Geology 46(9), 763–766. DOI: 10.1130/G45275.1.
- Seilacher A, Grazhdankin D, Legouta A 2003. Ediacaran biota: The dawn of animal life in the shadow of giant protists. Paleontological Research 7(1), 43–54. DOI: 10.2517/prpsj.7.43.
- Taylor PD, Vinn O, Wilson MA 2010. Evolution of biomineralization in ‘lopho- phorates’. Special Papers in Palaeontology 84, 317–333. DOI: 10.1111/j.1475-4983.2010.00985.x.
- Terleev AA, Postnikov AA, Tokarev DA, Sosnovskaya OV, Bagmet GN 2011. Cloudina-Namacalathus-Korilophyton association in the Vendian of Altai-Sayan Foldbelt (Siberia). in: Neoproterozoic sedimentary basins: stratigraphy, geodynamics and petroleum potential: Proceedings of the International Conference (Novosibirsk, 30 July – 02 Aug., 2011), 96–98.
- University of Edinburgh 2015. Complex skeletons evolved earlier than realized, fossils suggest. EurekAlert! Nov. 6, 2015.
- Warren LV, Quaglio F, Guimarães Simões M, Gaucher C, Riccomini C, Poiré DG, Tavares Freitas B, Boggiani PC, Noberga Sial A 2017. Cloudina–Corumbella–Namacalathus association from the Itapucumi Group, Paraguay: Increasing ecosystem complexity and tiering at the end of the Ediacaran. Precambrian Research 298, 79–87. DOI: 10.1016/j.precamres.2017.05.003.
- Watters WA, Grotzinger JP 2001. Digital reconstruction of calcified early metazoans, terminal Proterozoic Nama Group, Namibia. Paleobiology 27(1), 159–171. DOI: 10.1666/0094-8373(2001)027<0159:DROCEM>2.0.CO;2.
- Wood RA 2011. Paleoecology of the earliest skeletal metazoan communities: Implications for early biomineralization. Earth-Science Reviews 106(1–2), 184–190. DOI: 10.1016/j.earscirev.2011.01.011.
- Wood R 2017. Palaeoecology of Ediacaran metazoan reefs. in: Brasier AT, McIlroy D, McLoughlin N (eds). Earth System Evolution and Early Life: A Celebration of the Work of Martin Brasier. Geological Society, London, Special Publications 448(1), 195–210. DOI: 10.1144/SP448.1.
- Wood R, Curtis A 2015. Extensive metazoan reefs from the Ediacaran Nama Group, Namibia: the rise of benthic suspension feeding. Geobiology 13, 112–122. DOI: 10.1111/gbi.12122.
- Zhao Y, Vinther J, Parry LA, Wei F, Green E, Pisani D, Hou X, Edgecombe GD, Cong P 2019. Cambrian Sessile, Suspension Feeding Stem-Group Ctenophores and Evolution of the Comb Jelly Body Plan. Current Biology 29, 1112–1125. DOI: 10.1016/j.cub.2019.02.036.
- Zhuravlev AY, Wood RA 2008. Eve of biomineralization: Controls on skeletal mineralogy. Geology 36(12), 923–926. DOI: 10.1130/G25094A.1.
- Zhuralev AY, Liñán E, Gámez Vintaned JA, Debrenne F, Fedorov AB 2012. New finds of skeletal fossils in the terminal Neoproterozoic of the Siberian Platform and Spain. Acta Palaeontologica Polonica 57(1), 205–224. DOI: 10.4202/app.2010.0074.
- Zhuravlev AY, Wood RA, Penny AM 2015. Ediacaran skeletal metazoan interpreted as a lophophorate. Proceedings of the Royal Society B 282, 20151860, 1– 10. DOI: 10.1098/rspb.2015.1860.
