 Intelligent Design
Intelligent Design
More Cellular Roles Found for RNA
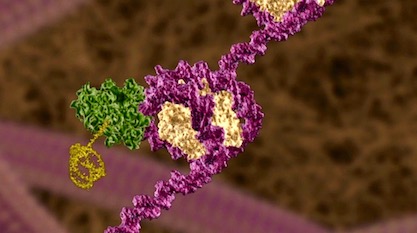
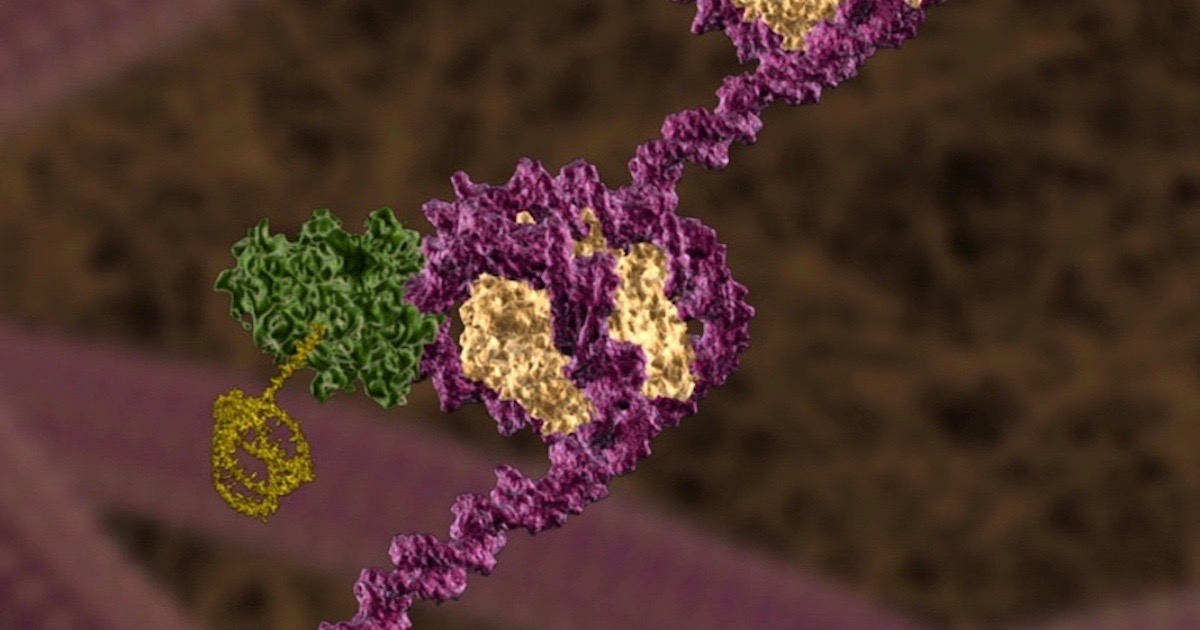
Previously relegated to servant tasks under the master DNA molecules, ribonucleic acids continue to surprise scientists with a multitude of important roles in the cell. Here are just a few making the news.
Orchestrators of Execution
Cells that go awry must be killed. Cell death is a carefully controlled process, involving two pathways: autophagy (“self-eating”) and apoptosis (programmed cell death). Apoptosis releases molecular machines called caspases that can cut up the components of a cell. Both processes are fairly well known, but a paper in PNAS reports the discovery of a “micro-RNA” that plays a critical role in the balance between the two. Welcome miRNA-378 to the captain’s lounge:
Muscle wasting and weakness can be observed under either physiological or pathological conditions, which are partly due to an imbalance between autophagy (“self-eating”) and apoptosis (“self-killing”). How microRNAs coordinate autophagy and apoptosis in the metabolic regulation of cell death remains largely unknown. This work identifies miR-378 as a critical component of metabolic checkpoints, which integrates metabolic information into an adaptive response to reduce the propensity of myocytes [muscle cells] to undergo apoptosis by enhancing autophagy and suppressing apoptosis via directly targeting phosphoinositide-dependent protein kinase 1 and Caspase 9, respectively. Our study highlights a crucial role of miR-378 in maintaining normal muscle homeostasis by orchestrating autophagy and apoptosis processes and provides a potential therapeutic target to treat myopathies. [Emphasis added.]
Notice the number of design words in this quote. The microRNA orchestrates. It integrates information. It coordinates the balance between two pathways. It targets other molecules. It balances and regulates, ensuring that checkpoints are respected. As a “critical component” with a “crucial role,” miR-378 deserves our respect and gratitude. Look what happens when it fails to perform its role in the “sophisticated” metabolic regulation of cell death: “our data suggest that inflammation-induced down-regulation of miR-378 might contribute to the pathogenesis of muscle dystrophy.” Remember all those Labor Day telethons by Jerry Lewis? Who would have thought that a cure might come by fixing a tiny little micro-RNA molecule? MicroRNAs are small, typically 20 to 24 nucleotides in length (NCBI). This one plays a big role for a small actor.
Site-Specific Activity
MicroRNAs were thought to act the same regardless of location. Zhang et al, writing in Nature Structural and Molecular Biology, found one that functions only with ORFs (open reading frames). This suggests the exciting possibility that some microRNAs “may use a translational quality-control-related mechanism to regulate translation in mammalian cells.”
RNA Organization
A couple of guys pictured in news from the University of Montreal use the risky phrase, “we now know” in their headline, “We now know how RNA molecules are organized in cells.” Well, perhaps they know a little bit about some RNA molecules, namely the messenger-RNA transcripts that ferry genetic information to the ribosomes for translation. For ease of understanding, the old Unlocking the Mystery of Life animation showed one of these mRNAs as a long, rigid molecule. Actually, due to intermolecular forces, mRNAs fold up and compact. Most biochemists believed the ends connected into a “closed-loop complex” that remained stable. Using super-resolution microscopy, lead author Daniel Zenklusen and his team were “very surprised” to find that a “decades-old dogma” is not correct:
It has long been thought that all messenger RNA, or mRNA, molecules acquire a specific conformation during protein synthesis: the two ends of the molecule coming together to form a stable so-called closed-loop complex. This new study shows that this long-standing model is oversimplified, according to Zenklusen and his team.
Their paper in Molecular Cell reveals that some mRNAs become very compact – so much so that they resist translation. They suspected this compaction regulates translation into proteins:
In collaboration with the laboratories of Olivia Rissland at the University of Colorado and Bin Wu at Johns Hopkins University in Baltimore, the UdeM scientists found that the messenger RNAs of cells can exist in many conformations but mostly as very compact molecules. This is most pronounced when protein synthesis is suppressed or messenger RNAs are sequestered to specific subcellular compartments such as stress granules….
Stress granules look like the useless clumps that pathologists find in the brain in neurodegenerative diseases. These clumps, however, appear to play active roles, down-regulating translation when a cell is under stress. The dense clumps of RNA and protein hinder translation, which could be a good thing in bad times. A 2016 paper in Trends in Molecular Biology indicates that stress granules must be important, because mutations in them can lead to degenerative disease.
The granules “are dynamic and show liquid-like behaviors but also contain stable substructures.” Perhaps they safeguard mRNAs and their related assisting machines when it’s not a good time for translation. “Stress granule formation modulates the stress response, viral infection, and signaling pathways,” the authors say. The ability of these granules to shift between solid and liquid states is undoubtedly related to their activity.
Solid-State Engineering
Speaking of liquid-like behaviors, another article suggested that liquid “droplet” formation is vital to the organization of cells. Not all components of a cell are neatly sequestered in organelles. Some float in the cytoplasm. How do those stay organized?
South Korean scientists are “Trying to Understand Cells’ Interior Design,” according to news from the Institute for Basic Science. What’s the opposite of dumb and careless? Watch their description:
How do you imagine the interior of our cells? Often compared to tiny factories, cells found smart and sophisticated ways to organize their ‘interior’. Most biological processes require cells to bring together their ‘employees’, such as proteins and nucleic acids (like DNA), at the right time. Scientists at the Center for Soft and Living Matter, within the Institute for Basic Science (IBS, South Korea), have explained how liquid-like droplets made of proteins and DNA form in vitro. Currently, there is a huge interest in understanding the molecular mechanisms behind the creation of such droplets, as it is linked to some human diseases, such as amyotrophic lateral sclerosis (ALS). The results, published as a featured article in Biophysical Journal, showed how much the sequence of DNA matters in the formation of such droplets.
The droplets consist of DNA, ATP and proteins (and RNA, which are mentioned in the paper). By experimenting on custom DNA strands, they found differences in the stiffness of DNA helices depending on whether they were composed of A-T base pairs or G-C base pairs. The former are more rigid; the latter, more fluid, allowing them to condense into droplets more readily. In addition, ATP facilitates the formation of the droplets.
So here we have another way cells can regulate their activity, taking advantage of solid- and liquid-phase transitions. This is all tied into the base sequence of the DNA or RNA.
This is a perfect platform to examine how the flexibility of nucleic acids affects liquid-liquid phase separation. “The most fascinating part is to imagine how cells may take advantage of this sequence-dependent information to guide and regulate liquid-liquid phase separation in vivo,” concludes [Anisha] Shakya, [the key contributor to the study.]
Perhaps we can now add a “phase code” to the information stored in the cell, which affects the activity of components floating around in the cytosol. Consistent with intelligent design predictions, the closer you look at life, the better it gets.
Image credit: Illustra Media.
