 Intelligent Design
Intelligent Design
Biophysicists Find Water Wires Are Biological Information Channels
Water conducts electricity; it can also conduct energy and information. Biophysicists are finding that “water wires” at the nanoscale fine-tune enzymatic actions — indeed, can be indispensable for function.
In a post here back in April, Evolution News shared a remarkable fact about dynein, one of the molecular machines that “walks” on microtubules. The scientist in that story theorized that water molecules bind to the stalk and help transmit kinetic energy via “water waves” from the reaction center, where ATP is spent, to the “feet” where walking takes place. The water molecules are so positioned as to create a virtual “tsunami” of energy from one end of the machine to the other, which causes the feet to move. Obviously, this requires very precise cooperation between the water molecules and the amino acids in the stalk. Now, other instances are coming to light of biological systems incorporating water molecules into their functional specificity.
How Water Wires Work
Biophysicists have long suspected that water molecules facilitate the passage of substrates through membrane channels. The purpose of membrane channels is to permit certain molecules through the cell membrane but prohibit others. This is called active transport, because normally molecules would move by diffusion (passive transport). Cells need to both attract the right molecules to go through the channel and authenticate them through the “selectivity filter.” Water can assist this process via electricity. Since H2O is bipolar, single water molecules in a chain, held together by hydrogen bonds, become a sort of “wire” through which ions can pass. Additionally, the fact that some amino acids are hydrophilic allows biological channels to attract water molecules to the exact positions inside the channel where they can assist the selectivity filter.
Building Water Wires by Design
In 2009, scientists in India constructed an artificial “peptide nanotube” just wide enough to hold a chain of water molecules. Chemistry World explains how achieving this in practice was harder than in theory:
Water behaves differently at the nanoscale, forming into single-file arrangements known as ‘water wires’. These are important in biology as they ferry protons through cell membranes, which is a crucial step in how most organisms produce energy.
The mechanism behind this proton transfer is the classic Grotthuss chain, where hydrogen bonding lets protons hop between water molecules very rapidly. But predicting how this mechanism affects a single line of molecules has been challenging. Now, a team led by Padmanabhan Balaram at the Indian Institute of Science in Bangalore has taken a first-hand look at how the molecules are configured, providing a useful template for future studies. [Emphasis added.]
The experiment in this early attempt did not attract water molecules to the sides of the channel but demonstrated the feasibility of doing so. In 2018, scientists from Cornell and Rensselaer made further progress in constructing artificial water wires. The news from Rensselaer, “Proof of Water Wires Motivated by a Biological Water Channel,” says that biological water channels called aquaporins provided the inspiration for their biomimetic experiments.
Aquaporins are proteins that serve as water channels to regulate the flow of water across biological cell membranes. They also remove excess salt and impurities in the body, and it is this aspect that has led to much interest in recent years in how to mimic the biochemical processes of aquaporins potentially for water desalination systems.
The experimenters built an artificial channel with stacks of imidazole, a ring-shaped nitrogen-based organic compound, and demonstrated for the first time that water molecules can be induced to stick to the sides of the channel in order to form a water wire. Cornell says that until then, water wires had been predicted theoretically but never seen.
“I would call this the first real observation of a water wire,” [Poul] Petersen said. “We’re not just seeing the oxygen [atoms], we see the protons, as well. It’s the first observation of the hydrogen bonding in a water wire.”
A key finding of this work was that the “net dipolar orientation of water molecules in confined channels induced specific polarization of the channel,” which drives the substrate through. The ability to mimic this action would be the “gold standard” for desalination technology, they say. But is this how water wires work in biological systems?
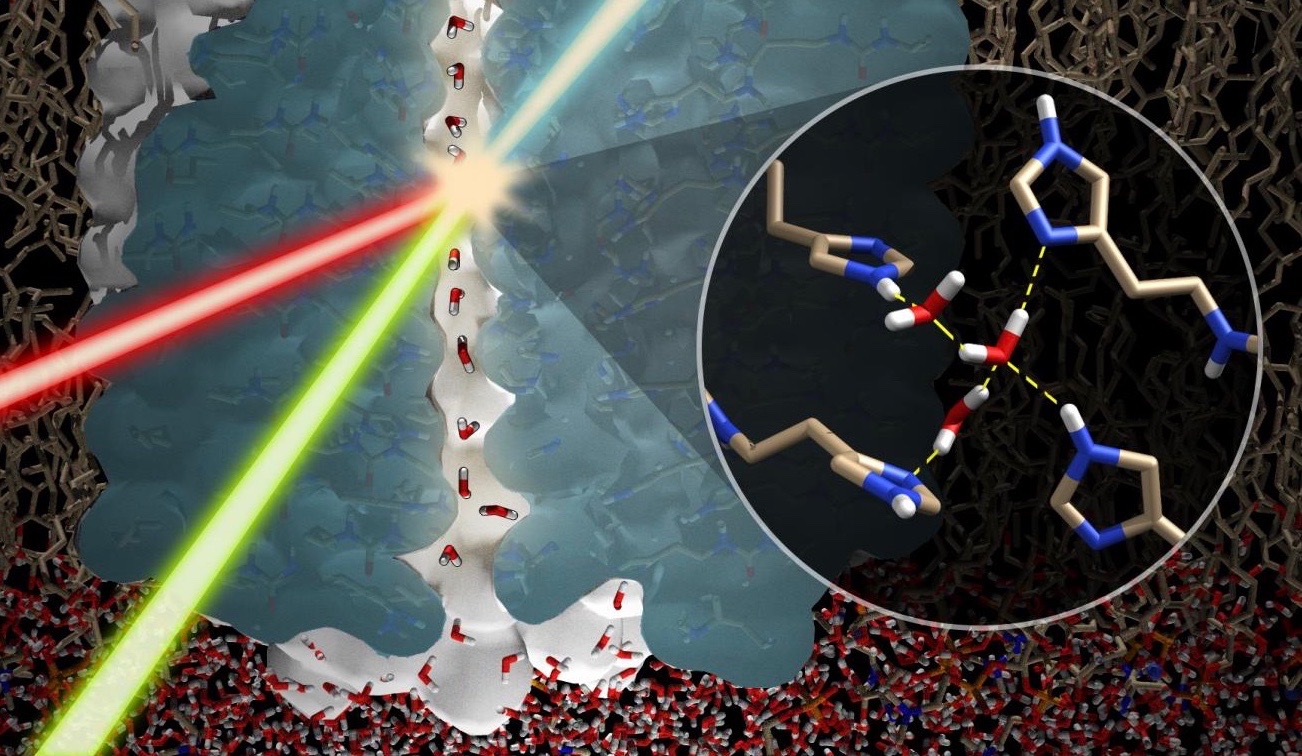
Super-Powerful Magnet Sees a Biological Water Wire for First Time
One challenge of observing biological water wires is peering inside the narrow confines of a membrane channel. Another challenge is slowing down the action. Hydrogen bond formation is predicted to change billions of times a second. This year, an international team used the ultra-high-intensity magnet at the National High Magnetic Field lab in Tallahassee, Florida, to achieve a breakthrough. Considering that the field energy of MRI machines in hospitals runs about 1 to 3 teslas (T), the power of the magnet at Tallahassee, 35.2 T, is truly astonishing. That is orders of magnitude stronger than the magnetic field of the earth or the sun.
Their paper in PNAS, “Functional stability of water wire–carbonyl interactions in an ion channel,” tells how they were able to pull off this feat in a well-characterized membrane channel named gramicidin A.
Water wires are critical for the functioning of many membrane proteins, as in channels that conduct water, protons, and other ions. Here, in liquid crystalline lipid bilayers under symmetric environmental conditions, the selective hydrogen bonding interactions between eight waters comprising a water wire and a subset of 26 carbonyl oxygens lining the antiparallel dimeric gramicidin A channel are characterized by 17O NMR spectroscopy at 35.2 T … and computational studies.
Gramicidin A (gA), discovered in 1939, is a linear peptide antibiotic produced by Bacillus brevis. Unlike most peptides, gA consists of alternating left- and right-handed amino acid residues. This gives it a spiral helical shape that penetrates the bacterium’s membrane, forming a pore that looks like a spiral staircase. This allows for the free passage of cations (positively charged ions) to neutralize the pH of the interior and exterior. “The antimicrobial activity,” the authors explain, “derives from its ability to form a transmembrane channel that is selective for monovalent cations.” It is a simple “channel” to use for studying the activity of a water wire.
The single file aqueous pore, terminated by the two L10 carbonyls, is only wide enough to host a single file of hydrogen-bonded water molecules: i.e., a water wire, as well as various monovalent cations ranging from Li+ to Cs+. The peptide planes of gA are nearly parallel to the pore axis, with odd-numbered carbonyls in both subunits oriented toward the bilayer center and even-numbered carbonyls toward the bilayer surfaces. Upon entering the water wire region of the channel, a cation interacts with only two waters and possibly pairs of carbonyl oxygens following a spiral path through the pore.
One surprising finding was that the hydrogen bonds in the wire are much more stable than predicted. Instead of changing in nanoseconds in the strong magnetic field, they lingered for milliseconds — six orders of magnitude longer.
The results reveal that selective pore-lining carbonyl oxygens form remarkably stable hydrogen bonds with waters in the wire, such that the water wire does not change its orientation on the millisecond NMR timescale. The stable orientation of the water-wire dipole also provides a simple explanation for the low affinity of the second cation binding site in this dimeric channel, despite a separation of ∼24 Å from the first binding site at the opposite end of the pore.
The dipole formed by the 24-fold difference in binding affinity forces the cation down the channel. The water molecules are placed at the precise positions to provide optimum stability.
The water wire itself has a stability gradient from the negative end of the electric dipole to the positive end, based on optimal hydrogen bonding of the waters at the negative end of the electric dipole. The water interactions at this end of the dipole over the first three waters of the water wire are particularly stable.
Is there a reason why the hydrogen bonds need to be stable? Yes; the timing of passage of cations requires that the binding sites not flip too quickly. You can skip this passage after the first sentence unless you like the details:
Water wires are critical biological assemblies supported by membrane proteins for the purposes of transporting charge and ions across membranes. The present unique high-resolution characterization of the gA water wire provides insights into the dynamics, structure, and functional mechanism of this ion channel. In liquid crystalline lipid bilayers, the gA water wire and its associated electric dipole are stable on the millisecond timescale, both with and without cations present. Upon single cation occupancy, the influence of the electric dipole extends even further into the terminal region of the second subunit than in the absence of cations. The K+ interactions upon double occupancy do not result in symmetrization of the channel; however, there are significant impacts on water carbonyl interactions in the pore and hence on the resultant electric dipole that stabilizes the high affinity cation binding state. Furthermore, under double occupancy, the cations in the high- and low-affinity sites do not exchange sites at their respective ends of the channel on the millisecond timescale. Such exchange would flip the water wire and its associated electric dipole, resulting in averaging of the 17O spectral frequencies of the two subunits into a single value. Consequently, these cation binding sites are also stable at least on the millisecond timescale. Indeed, the water-wire orientation and its associated electric dipole dictates the high-affinity and low-affinity sites of the dimer.
In short, the spacing of all the amino acids and water molecules is optimized for function. The first ion is sucked in, forming its stable hydrogen bonds with the water, so that the second ion entering the channel doesn’t flip the dipole over and break the flow. That requires precision foresight both positionally and temporally. And this is one of the simplest examples found in a bacterium! It is fair to expect even more optimization will be found in future studies of water wires in more complex membrane channels. Do the authors think this is intelligently designed? They almost say so:
The profound influence of the water wire in this model system and the stable water–carbonyl interactions illustrate the significance and functionality for such wires in many channels and materials. In particular, the stability of the water wire and its electric dipole suggests that its influence in many other systems could be more significant than generally recognized. To achieve such an understanding, unique 17O spectroscopy as reported here at a field strength of 35.2 T demonstrates the exquisite sensitivity to the chemical environs surrounding oxygen sites where so much of biological chemistry takes place.
Their awe at this system might explain their failure to attribute it to evolution.
