 Evolution
Evolution
 Intelligent Design
Intelligent Design
Compact Factory Optimizes Shape for Efficiency — A New Level of Intelligent Design in Life
A well-designed factory will consider more than the functions that must be performed. To optimize productivity, engineers will design up front how the functions fit together in space. The shape of the factory, to maximize efficiency, will have the functional units arranged such that the output of one unit feeds easily into the input of another. This minimizes delays and maximizes production. What should one think about finding such design in a protist — a single-celled organism?
OxPhos, ETC
Among sets of cellular machines that coordinate their functions, certainly one of the finest examples is the metabolic machinery operating in mitochondria. This machinery is composed of five complexes labeled with Roman numerals I through V, with Complex V being the famous ATP synthase rotary engine. The set of machines, each one irreducibly complex, performs “oxidative phosphorylation” (OxPhos) through an “electron transport chain” (ETC). Electrons from food are transferred by enzymes through Complexes I, II, III, and IV to pump protons into the intermembrane space. This creates the proton motive force that drives ATP synthase. Similar processes keep everything on the planet alive, including us. The oxygen we inhale is the final electron acceptor. In a real sense, oxygen serves the purpose of helping run ATP synthase for life’s energy requirements.
A beautiful animation of the cellular factory in mitochondria was released by Science X on YouTube.
In less than four minutes, viewers can watch how the proton motive force is generated by Complexes I-IV and their associated enzymes to power ATP synthase. The visualization, produced by the European Research Council, helps laypersons understand the flow of activity without getting hung up on the molecular jargon. I suggest that you watch the animation now, then we will discuss new findings about this remarkable and well-organized factory. The upshot is that the system is optimized not only by the arrangement of the five Complexes, but by the physical structure, or shape, that the arrangement produces. And this was discovered in a microbe!
Tetra-what?
The discovery was published in Nature by a European team led by Alexander Mühleip. Because the paper is open access, readers can view with awe the colorful figures showing the arrangement of machines in the factory. The main figure was also reproduced by the Science for Life Laboratory, along with the animation. The headline says, “A massive supercomplex induces membrane curvature for cellular respiration.”
And do they mean massive and super! The paper says this supercomplex has “150 different proteins and 311 bound lipids, forming a stable 5.8-MDa assembly.” 5.8 megadaltons is the equivalent of 5,800,000 atomic mass units. Irreducible complexity, anyone?
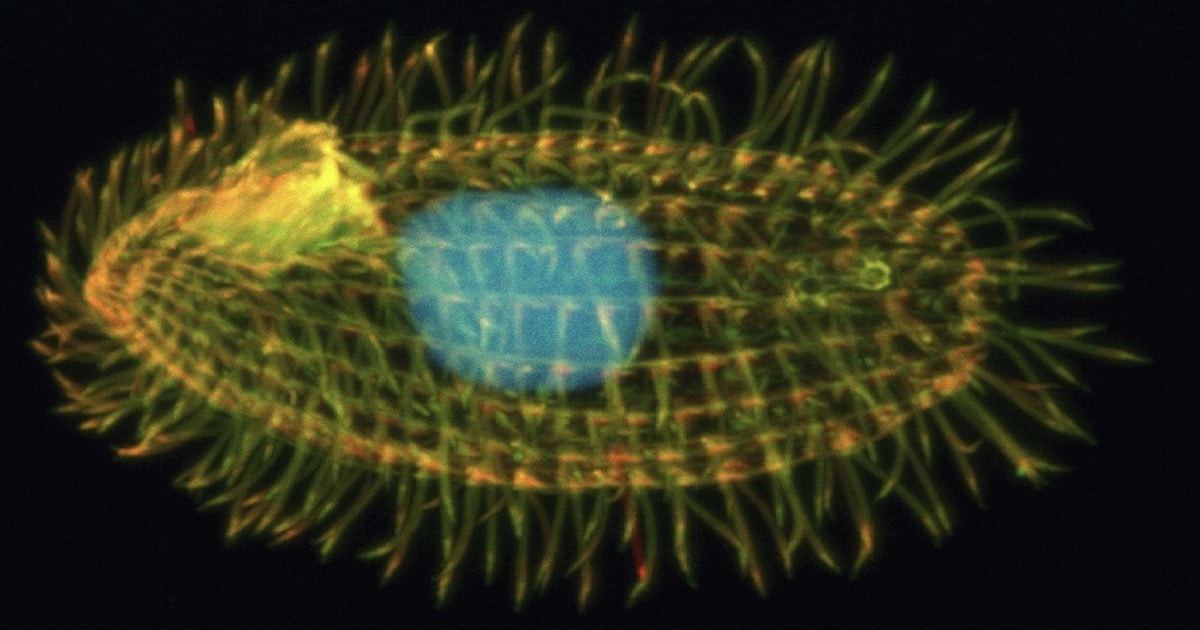
Such a system must be part of the most advanced organism at the top of the evolutionary ladder, right? No; they found it in a protist — a one-celled organism found in warm little ponds. Their model organism is named Tetrahymena thermophila, a member of protists called ciliates, which includes the well-known Paramecium and others shown in high-school biology textbooks or under class microscopes, like Stentor and Vorticella. Ciliates, busily whipping their cilia for movement, are found in almost every environment on earth, from arctic sea ice to hot springs. Each cilium, another of Michael Behe’s icons of irreducible complexity (IC), is powered by ATP, and T. thermophila is covered with cilia. Each free-living cell can have up to a thousand of them.
Structural Foresight
The main point of the study was that the spatial arrangement of complexes in the mitochondria of T. thermophilaphysically alters the shape of the factory for optimal productivity. The figure shows how the complexes are tightly packed together like pieces of a puzzle. The packing induces a bend in the cristae (the dual membranes inside the mitochondria) that forces the membrane into a tubular shape which, for this hot-spring-loving protist, best controls the proton flow:
They form a massive 5.8 megadalton supercomplex of 150 proteins with at least 300 transmembrane helices and 311 lipids. Owing to subunit acquisition and extension, Complex I binds a dimer of Complex III that is tilted by 37 degrees. Complex I also associates with the Complex IV dimer, generating a gap that serves as a binding site for Complex II.
The study demonstrates that this assembly is crucial to the shaping of the bioenergetic membrane. One of themost intriguing findings is that a subunit of Complex IV called COX3 is split in two. The fragmentation occurs on the genetic level, and then each fragment is extended, contributing to some of the interfaces between complexes. [Emphasis added.]
Skip Ad
The next sentence says, “The gain of function for inter-complex contacts represents an evolutionary mechanism, showing how neutral molecular complexity can become beneficial.” It’s permissible to skip over that narrative gloss. Another interruption in the story, like those pesky ads on YouTube that interrupt your viewing, appears in the paper: “Our findings highlight how the evolution of protein subunits of respiratory complexes has led to the I–II–III2–IV2 supercomplex that contributes to the shaping of the bioenergetic membrane, thereby enabling its functional specialization.” One doesn’t have to click the ad or buy the product.
The other four mentions of evolution add no value. Here’s one more that can be skipped over to get to the subsequent, more informative, paragraph:
The findings highlight how the evolution of protein subunits of respiratory complexes has led to the supercomplex assembly, which actively contributes to mitochondrial membrane curvature induction that is necessary for proper mitochondrial function.
This way, the supercomplex shapes the macroscopic architecture of mitochondria, ultimately optimizing ATP synthesis. Therefore, respiratory supercomplexes have not only an enzymatic but also a structural function of shaping the membrane, and both together support energy conversion and provide fuel for life.
Human Mitochondria
Unlike this protist, higher eukaryotes do not have tube-shaped cristae. This does not mean that our cristae are not also structured in 3-D space for optimization. The heat-loving microbe has special needs. The paper suggests a general principle at work: shape contributes to productivity.
Our results indicate that cristae shaping involves both the respiratory supercomplex and the ATP synthasethat together generate membrane tubulation. Although the coiled ATP synthase rows fix the helix diameter at 130 nm, supercomplexes serve the function of confining a narrow crista diameter of around 40 nm, which allows tight packing of cristae, thereby increasing the surface area of the bioenergetic membrane. This membrane-shaping organization of the respiratory supercomplex is markedly different from the mammalian homologue, which resides in the flat crista regions. Furthermore, because every crista represents an independent functional compartment, restriction of the crista diameter by the respiratory supercomplex probably serves to minimize the volume, thereby potentially contributing to higher local concentration of electron carriers, ensuring that proton translocation results in an increased local proton motive force, ultimately optimizingconditions for ATP synthesis. This is consistent with the observation that mutant yeast strains with large, balloon-like cristae display respiratory defects. Thus, our findings show how respiratory supercomplexes together with other factors can organize the architecture of the bioenergetic membrane, providing a mechanism for enabling its functional specialization.
Indeed, an earlier paper in PNAS showed that the cristae in yeast and mammals are folded into their characteristic wavy form for a purpose. The angle of ATP synthase dimers induces a “pinch” shape in the cristae that draws in protons from the intermembrane space like a funnel to keep the ATP synthase rotors spinning at optimal levels, while simultaneously increasing the surface area for the engines. (That’s the only drawback in the animation; those rotors spin really, really fast in actuality — almost 8,000 rpm!)
Induced Shaping: Another Design Rule
Another recent paper found a case of induced shaping. In Science Advances, an American team found that some intrinsically disordered proteins, which might seem awkward from an intelligent design perspective, actually illustrate a benefit in the disorder. “Specifically,” they wrote, “repulsive interactions among disordered domains drive convex bending, while attractive interactions drive concave bending, creating membrane-bound, liquid-like condensates.” They call this one of a set of “design rules for membrane bending in disordered proteins.”
On the basis of our findings, interactions between these domains could help to stabilize the complex architecture of the nuclear pore, which contains both convex and concave curvatures. Inspired by these examples and the growing recognition of the role that disordered proteins play in curving membranes, the design rulesidentified in the present study have broad implications for our understanding of the diverse mechanisms by which protein networks shape biological membranes.
Shape induction constitutes a new level of design for optimization. The physical properties of the complexes in mitochondria of T. thermophila — their charge distribution, attraction or repulsion to water, and the orientations of their inputs and outputs — have been shown to induce curvatures in the membranes that serve to increase productivity while protecting the complexes from thermal damage. Each complex is sophisticated enough on its own to illustrate design. Did it not require superintelligence to arrange a supercomplex of IC machines to optimize productivity? The stuff of life looks super-engineered.
