 Evolution
Evolution
 Intelligent Design
Intelligent Design
Newly Discovered War Machines in the Immune System
An armored terrorist lurks in the city. Suddenly, thousands of pieces of sticky rope fly at him from all directions. They bind together, immobilizing him in a net. The net dissolves the intruder’s armor, and simultaneously signals for miniature robotic snipers who land on the net, using it as a scaffold. They fire armor-penetrating bullets through the net and into the terrorist’s compromised armor. Reinforcements install kill switches inside his body, forcing the terrorist to commit involuntary suicide.
Something like that describes a newly discovered molecular machine that helps fight infectious pathogens in our body cells. The news from Yale University says:
Yale scientists have discovered a family of immune proteins, which they describe as a “massive molecular machine,” that could affect the way our bodies fight infection. [Emphasis added.]
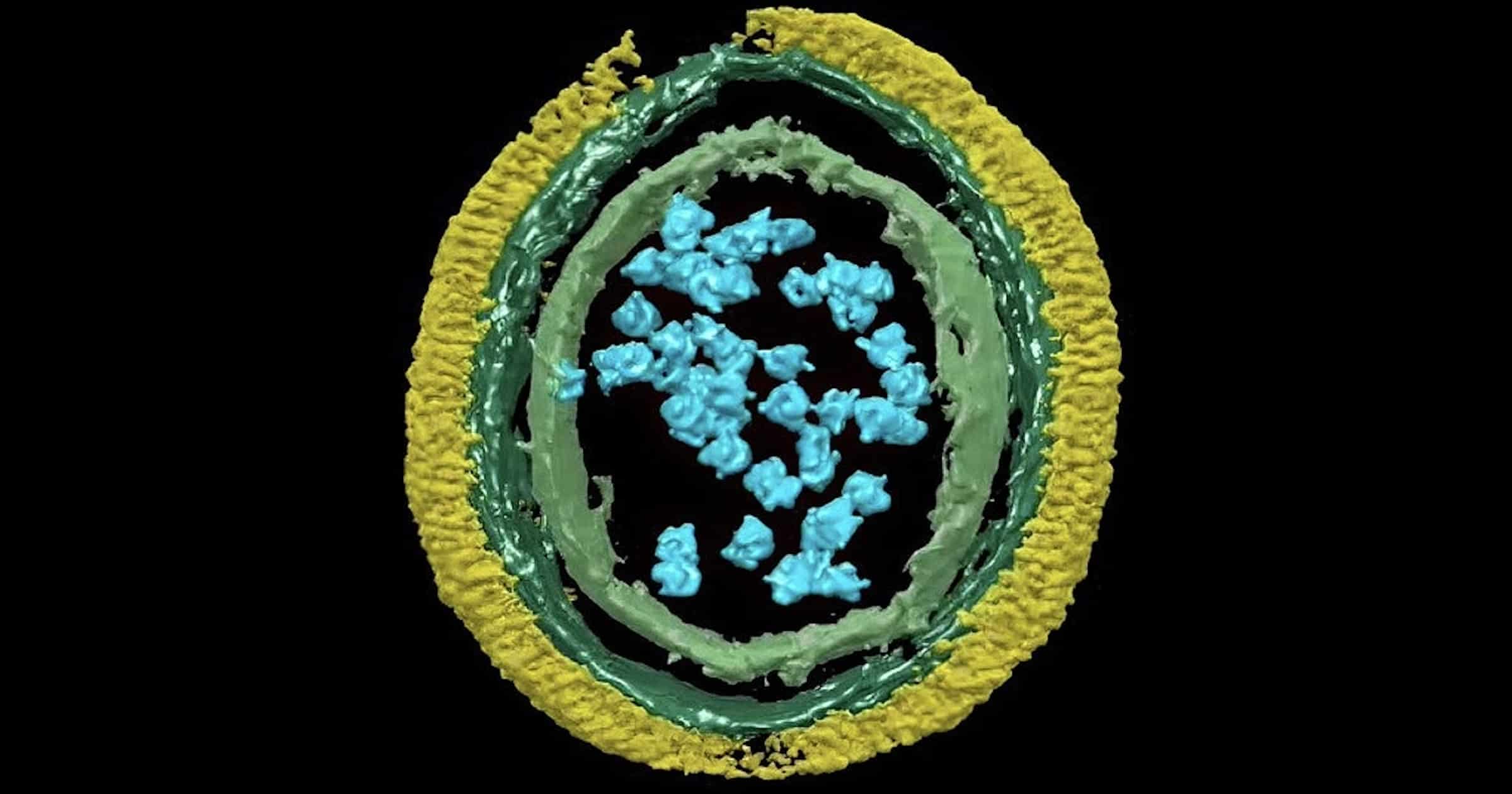
The immune proteins forming the net around the pathogen are called guanylate binding proteins, or GBPs. They have been known for a decade, but their mode of operation was only recently uncovered by Yale researchers. A short video shows these GBP1 proteins (the sticky ropes) as yellow pillar-shaped dimers rushing in, unfolding and linking up, surrounding the outer membrane of a bacterium (its armor). In short order the bacterium is surrounded with an inescapable straitjacket. There can be up to 30,000 of these proteins enclosing the pathogen in a type of body bag.
“What we found is among the most impressive examples of a biological machine in action that I’ve ever seen,” said John MacMicking, a professor of microbial pathogenesis and of immunobiology at Yale, and an Investigator of the Howard Hughes Medical Institute. MacMicking is senior author of the study.
The bacterial cell wall armor is no match for the immune system’s armor-piercing bullets. Even bacteria able to modify the lipopolysaccharides (LPS) that comprise the outer membrane (OM) have no chance. GBP1 knows all the configurations.
Human GBP1 still targeted cytosolic Stm [Salmonella enterica serovar Typhimurium] irrespective of bacterial size, shape, motility, or OM composition; the latter spanned LPS chains of different length, charge, and chemical structure. Such broad ligand promiscuity may help GBP1 combat gram-negative pathogens that modify their LPS moiety in an attempt to evade innate immune recognition and antimicrobial killing.
With the pathogen’s armor covered, the GBP proteins work to disentangle the lipopolysaccharide threads of the outer membrane. Having detected the help signal, reinforcements come in, firing caspase-4 grenades and interferon-γ kill switches into the bacterium, forcing it to commit pyroptosis, a form of programmed cell death.
“We are literally observing Mother Nature at work, looking at how these proteins operate in 3-dimensional space and at a particular location,” said MacMicking. “In just a few minutes they unfold and insert into the bacterial membrane to form a truly remarkable nanomachine and innate immune signaling platform.”
The bacteria coated with GBP straitjackets can be as small as 750 billionths of a meter (nanometers). The scientists found that this body-bag method works on bacteria regardless of shape. It works on viruses, too.
Imaging Design in Detail
This discovery was only made possible by recent advances in imaging technology. With cryo-electron microscopy, the researchers were able to “slice” whole live cells that had been quick-frozen. The resulting slices were assembled into tomograms, giving glimpses of heretofore unseen realities at work inside our body cells.
Our immune system mobilizes numerous proteins to detect viruses and bacteria — and to bring them under control. But until recently, limits to research technology have thwarted scientists’ understanding of how to prevent different pathogens from occupying and replicating within specific parts of our cells in the first place.
Harnessing the latest cryo‐electron microscopy techniques to look inside human cells, researchers at the Yale Systems Biology Institute have identified a family of large immune proteins that assemble into a massive signaling platform directly on the surface of microbial pathogens.
The researchers say they found thousands of GBPs building what amounted to a coat of armor (GBP1 coat complex) around the bacteria, allowing other defense proteins to recognize and kill encapsulated bacteria as well as mobilize immune cells for protection.
This reinforces an ID expectation that the more detail revealed, the more the design evidence becomes apparent. Evolution may look plausible from afar, but the angel is in the details.
How Reinforcements Are Called
After the bacterium is immobilized, the GBP1 straitjacket becomes a scaffold for snipers to dismantle the intruder’s armor. The GBP family of proteins serve not only as the sticky ropes coating the intruder and disrupting its armor; they are also equipped with radios to call in the snipers and bomb squad. These proteins install the kill switches.
Thus, insertion of human GBP1 seems to disrupt lateral LPS-LPS interactions to compromise OM integrity. This not only activates the caspase-4 inflammasome pathway but allows the passage of small antimicrobial proteins such as APOL3 to directly kill pathogenic bacteria.
Human GBP1 was found to be “obligate for initiating the entire signaling cascade,” the scientists found via knockout experiments. It’s the captain in command.
Irreducible Complexity in Peace and War
ID advocates enjoy the examples of irreducible complexity (IC) in peacetime: the ATP synthase motor, kinesin, and the DNA translation mechanism. But when intruders threaten the life of a cell or its host organism, IC can fight with lethal intensity in an “all hands on deck!” war campaign. Its armed forces are always at the ready.
An emerging paradigm for innate immune signaling cascades is the higher-order assembly of repetitive protein units that generate large polymers capable of amplifying signal transduction. Our results identify human GBP1 as the principal repetitive unit, numbering thousands of proteins per bacillus, that undergoes dramatic conformational opening to establish a host defense platform directly on the surface of gram-negative bacteria. This platform enabled the recruitment of other immune partners, including GBP family members and components of the inflammasome pathway, that initiate protective responses downstream of activating cytokines such as interferon-γ. Elucidating this giant molecular structure not only expands our understanding of how human cells recognize and combat infection but may also have implication for antibacterial approaches within the human population.
Isn’t it nice to know that “eukaryotes have evolved compartment-specific immune surveillance mechanisms that alert the host to infection and recruit antimicrobial proteins that help bring microbial replication under control”? Actually, Charles Darwin never proved that his proposed mechanism of natural selection was capable of creating anything beyond simple variation within a species. His use of rhetoric and the analogy of domestic breeding was recognized even by his contemporaries as a mere suggestive hypothesis lacking scientific demonstration.
Robert Shedinger shows this in Darwin’s own words in the new book Darwin’s Bluff. Aware that the Origin of Species was a “mere abstract” falling short scientific standards, Darwin promised a “big book” with the evidence. But he never published one. Why? Shedinger suggests he knew the evidence was lacking, and he was afraid of criticism. Instead, he relied on friends to promote his views. Darwin’s friends ran with “natural selection” as an all-purpose can opener to explain nature without an intelligent designer, using imagination and storytelling instead of hard evidence. In my experience reading the best of neo-Darwinian explanations, that’s still all they have to offer. Demonstration of selection’s alleged creative power is lacking, especially for irreducibly complex “massive molecular machines” like this one.
The discovery of a multi-component system able to mount a coordinated response to a threat speaks instead of Foresight: preparedness for a future eventuality. Darwin’s mechanism has no foresight or goal. At best, it can only preserve what it already has. Our uniform experience with foresight is that it is a capability of designing intelligence. That is Undeniable.
