 Intelligent Design
Intelligent Design
Out of One Cell, Many Tissues — But How?
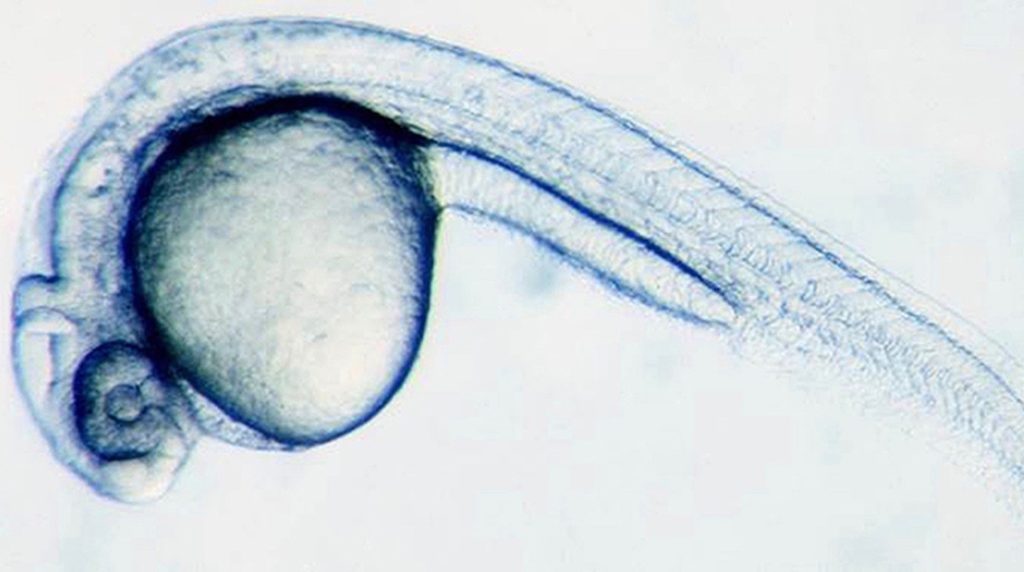
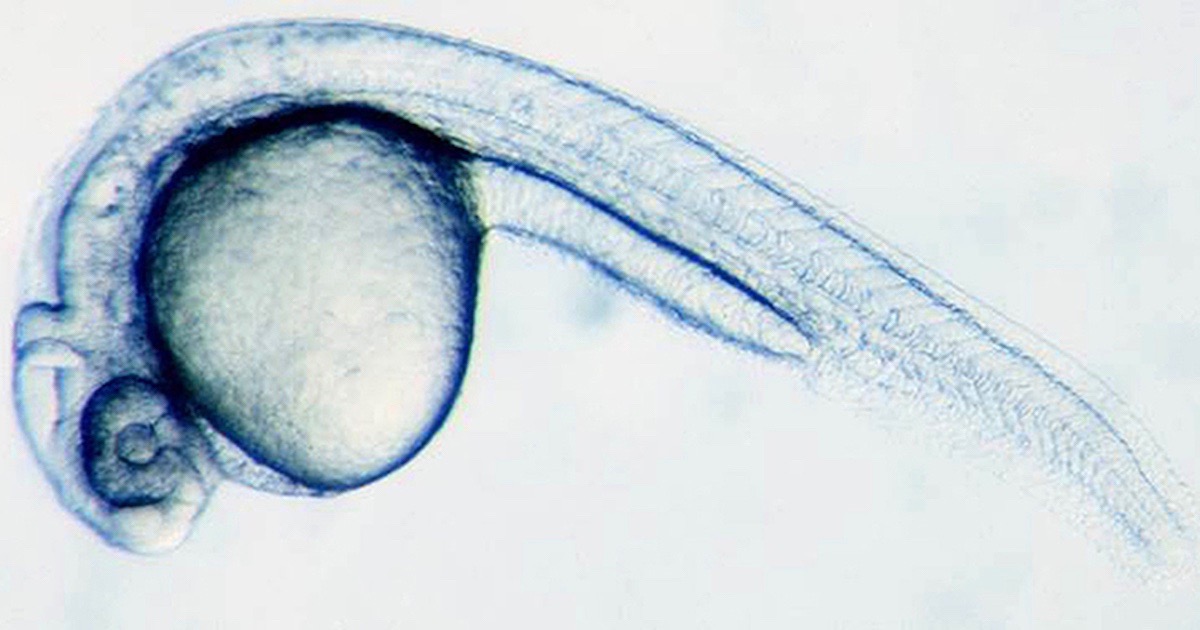
News from Harvard University shows how a zebrafish embryo develops from a single cell into thousands of cells. When you think about all that must happen in the right order to make this possible, it is truly remarkable. It’s one of the most carefully orchestrated sequences of events we know of, and yet it happens in the development of every multicellular organism.
Whether a worm, a human or a blue whale, all multicellular life begins as a single-celled egg.
From this solitary cell emerges the galaxy of others needed to build an organism, with each new cell developing in the right place at the right time to carry out a precise function in coordination with its neighbors.
This feat is one of the most remarkable in the natural world, and despite decades of study, a complete understanding of the process has eluded biologists. [Emphasis added.]
Paul Nelson showed how ontogeny recapitulates design in the video, “How to Build a Worm.”
Each stage — each cell division — though individually distinct and oblivious to the whole, contributes to a process that coordinates and choreographs the assembly of many separately necessary parts to achieve a functional end. “It’s the quintessential end-directed or teleological process in nature,” he says. Ann Gauger compared it to “a Bach fugue that splits and weaves many voices into one.”
Two Larger Organisms
At the time of Nelson’s video, scientists had only accounted for all the developmental steps in Caenorhabditis elegans, a one-millimeter-long roundworm with about a thousand cells. This year, scientists at Harvard have watched the development of two larger organisms in detail.
Now, in three landmark studies published online April 26 in Science, Harvard Medical School and Harvard University researchers report how they have systematically profiled every cell in developing zebrafish and frog embryos to establish a roadmap revealing how one cell builds an entire organism.
Using single-cell sequencing technology, the research teams traced the fates of individual cells over the first 24 hours of the life of an embryo. Their analyses reveal the comprehensive landscape of which genes are switched on or off, and when, as embryonic cells transition into new cell states and types.
Together, the findings represent a catalog of genetic “recipes” for generating different cell types in two important model species and provide an unprecedented resource for the study of developmental biology and disease.
Using new experimental and computational techniques, the scientists monitored gene expression profiles at each stage, one cell at a time, for 200,000 cells. Three teams monitored two well-studied model species, the zebrafish and the western claw-toed frog, Xenopus tropicalis. Embedded videos show what they saw. Harvard molecular biologist Alexander Schier remarked, “It is almost like going from seeing a few stars to seeing the entire universe.”
The teams sampled cells at various stages and sequenced them to monitor what the messenger RNAs were doing. And by creating mutants, the scientists could also watch what happens when the canonical pathway is disrupted.
Unexpectedly, the groups independently found that at the single-cell level, gene expression was the same in mutants and wildtype, despite the loss of an essential signaling pathway. The proportions of different cell types, however, changed….
When Klein, Kirschner, Megason and colleagues compared cell-state landscapes between zebrafish and frog embryos, they observed mostly similarities. But their analyses revealed numerous surprises as well. One such observation was that genes marking cell states in one species were often poor gene markers for the same cell state in the other species.
In several instances, they found that the DNA sequence of a gene — and the structure of the protein it encodes — could be nearly identical between species but have very different expression patterns.
“This really shocked us, because it goes against all the intuition we had about development and biology,” Klein said. “It was a really uncomfortable observation. It directly challenges our idea of what it means to be a certain ‘cell type.’”
The findings suggest that the underlying control system knows how to reach the target in spite of differences in expression patterns along the way. Another surprise was that the branching-tree pattern of cell divisions and cell fates appears overly simplistic.
In another striking finding, the teams observed that the process of cell differentiation into distinct cell types — which is commonly thought to occur in a tree-like structure where different cell types branch off from a common ancestor cell — can form “loops” as well as branches.
For example, the neural crest — a group of cells that give rise to diverse tissue types including smooth muscle, certain neurons and craniofacial bone — initially emerges from neural and skin precursors, but is well-known to generate cells that appear almost identical to bone and cartilage precursors.
The new results suggest that similar loops might occur in other situations. That cells in the same state can have very different developmental histories suggests that our hierarchical view of development as a “tree” is far too simplified, Klein said.
We know humans are capable of this kind of design. A foreman can repurpose materials or workers depending on circumstances, as long as he keeps in mind what the blueprint calls for. But how does an embryo know this?
Reaching the Goal, Regardless of the Path
All three teams identified cell populations reaching intermediate “decision making” states. They watched as “cells appeared to go down one developmental trajectory but then changed their fate to another trajectory.” Again, this violates deterministic programming that can only reach the target one way. Something knows how to keep the final goal in mind, regardless of the path.
Klein, Megason, Kirschner and colleagues made a related observation that, early in development, some cells activated two distinct developmental programs. Though those intermediate cells would eventually adopt a single identity, these discoveries add to the picture of how cells develop their eventual fate and hint that there may be factors beyond genes involved in directing cell fate.
“With multilineage cells, we have to start wondering if their final fate is being determined by some selective force or interaction with the environment, rather than just genetic programs,” Kirschner said.
Alex Schier’s team, which worked on one of the zebrafish papers, was surprised at this flexibility of the embryo. News from the University of Basel tells how they found that outcomes of cell fates can be altered by circumstances:
The results show that the genetic program that a cell follows on the way to maturity is by no means set in stone. “It seems that the developmental path of a cell is more flexible than we previously expected”, says Alex Schier. So far, it was assumed that developing cells follow a predetermined path, like marbles rolling down a hill until they stop at their predestined place. The study now suggests that signals from the environment can have such a strong influence on the cells, that they leave the initial trajectory and change their path, thus taking on a new identity.
This finding calls to mind navigators in the space program, who can make course corrections en route to a target when they encounter unexpected circumstances. There was a famous case on the Cassini mission, for example, when mission planners found a defect in the communications relay between the orbiter and the Titan probe. By using the gravity of Titan to make an additional orbit, they changed the angle of the relay, and the data from the Titan landing mission was saved.
Hardly a Mention of Evolution
The three papers in Science are located here for zebrafish gene expression landscapes, and here for zebrafish developmental trajectories, and here for Xenopus frog gene expression dynamics. Only the third paper mentions evolution, noting the possibility that cell identities appear to be decoupled from genes across evolution. “We found that this expression plasticity is independent of variation in protein sequence itself, surprisingly decoupling a gene’s structure from its expression pattern in the embryo across evolution.”
Summarizing these papers, Elizabeth Pennisi in Science described how the three teams compared notes.
When Klein, Kirschner, and Megason compared the results for the frog and the zebrafish, they found surprising differences. For example, the developmental routes of certain cell types varied by species. And although the activity of key transcription factor genes was similar in common cell types, the activity of other genes in some cell types differed more than the researchers expected between the two species.
Both zebrafish teams also tracked gene activity in fish that had a mutation expected to seriously disturb development. The two groups’ different mutations completely eliminated specific cell types — presumably those directly affected by the disrupted gene — but most other cells differentiated almost normally. This “is just the tip of the iceberg,” in terms of analyzing the developmental effects of mutations, Arendt says.
Conclusions
Paul Nelson’s case for intelligent design is strengthened by these new studies. Developmental programs can now be seen as more flexible and robust than previously thought. Embryos can make “course corrections” en route to reach the target. And yet, “Ten, 20 years from now, we can still be sure zebrafish and frogs are going to develop according to the same patterns,” Klein predicts. The target is fixed, even if the way to reach it is flexible.
It’s also worth noting that evolutionary theory played almost no part in these research efforts. Only one of all the sources cited above even mentioned evolution; and there, it was only a matter of suggestion for future study. In fact, the authors of the Xenopus paper were rather surprised that a gene’s expression pattern could be decoupled from its structure. What does that do to the old neo-Darwinist mutation/selection theory?
The video with Paul Nelson shows artists and architects working on distant goals, performing individual tasks that contribute to reaching the goal. A bricklayer or plumber doesn’t need to know what the finished building will look like. The architect envisioned the target in his mind, according to his skill and knowledge of the requirements. He turns the job over to a foreman who brings it about. We can all think of dozens of examples of this goal-directed activity. When we see analogues in the construction of living things, we can make a robust inference, based on causes we know, that what Doug Axe calls “functional wholes” show the clear imprint of intelligent design in their conception, and intelligent guidance in their manufacture.
Photo: Zebrafish embryo at 28 hours, via University of Basel.
