 Intelligent Design
Intelligent Design
Three Flagellum Updates Amplify Behe’s Challenge to Darwinism from Irreducible Complexity
Depictions of the bacterial flagellum in 1996, the year Darwin’s Black Box was published, and in 2002, when Unlocking the Mystery of Life brought it to the screen in animation, look crude by comparison to the latest ones that show all the parts in near-nanometer resolution.* But they were sharp enough to motivate Michael Behe’s “Revolutionary” argument for intelligent design from irreducible complexity. Three new papers do nothing to undermine that design inference, despite some of the researchers’ appeals to evolution. Instead, the papers add new details about the precision working of these machines, unknown 15 years ago, that accentuate the argument for design.
Stators and Speed
The first paper, in the Proceedings of the National Academy of Sciences (PNAS), was edited by Howard Berg. He’s the Harvard expert on flagella who was mentioned briefly in Unlocking as having called the flagellum the “most efficient machine in the universe.” In this paper, researchers from Oxford, Japan, and China wanted to know if the rotation speed was affected by the number of stator units. Previous work had suggested that flagella could rotate at full speed independently of stator subunit count. Through a series of precise measurements on flagella from different bacterial species mutated to vary the number, here’s what they found:
The bacterial flagellar motor is a rotary molecular motor responsible for swimming, swarming, and chemotaxis in many species of bacteria. It generates torque by interactions between a rotor 50 nm in diameter and multiple stator units. We overturn the prevailing understanding of how stator units interact with each other by showing that motor speed is dependent on the number of stator units even at high speed near zero torque. [Emphasis added.]
The relationship held for both the sodium-ion type (Na+) and hydrogen-ion type (H+) motors. The team could not explain the discrepancy with previous findings, but offered an evolutionary hypothesis for the difference between clockwise (CW) and counterclockwise (CCW) measurements:
We speculate that the CCW torque–speed relationship may indicate selection pressure for high power output, as it combines high torque and high speed at viscous drag coefficients similar to those experienced in the bundle of a swimming E. coli cell. By contrast, the linear relationship seen in CW rotation is easier to achieve in mechanistic models and may represent a lack of selection pressure given that CW rotation is not typically used to propel swimming.
This evolutionary “speculation” about “selection pressure” could easily be recast as a functional design requirement. In a stick-shift car, for instance, you may have several forward gears but only one reverse gear. Other than this one paragraph, the authors said nothing about evolution. They did, however, suggest an intriguing possibility that slower flagella might result from mutations or other degrading processes:
If a fully functional unit rotates at 240–300 Hz, lower speed levels might represent partially functional units. Speculations as to the possible nature of these include units attached to the peptidoglycan at suboptimal orientations or distances from the rotor and half-functional units with one channel blocked, misfolded, or otherwise misassembled.
Shape-Switching Mechanism in the Propeller
As mentioned in Unlocking, bacteria can reverse the direction of rotation rapidly. The propeller itself, though, must also quickly adjust its protein subunits to “supercoil” in the opposite direction. Here’s news: the supercoiling is required for function. That fact inspired some machine language, found in this paper in Nature Communications:
The bacterial flagellar filament has been intensively studied for many years. It has served as an enlightening system for understanding how a protein polymer composed of a single protein, flagellin (except for the cap protein at the end that acts as an assembling chaperone) switches among different states to supercoil. This supercoiling allows the rotating filament to behave as an Archimedean screw and produce thrust. The filament can adopt different conformational states due to mechanical forces, such as when the motor switches the sense of rotation, allowing the bacteria to swim forward, backward, in a screw-like fashion and to tumble.
As Behe had pointed out, mutations to essential parts of an irreducibly complex system can lead to loss of function:
With the motor linked to sensory receptors, the bacteria are capable of moving towards nutrients and away from dangerous environments, resulting in a significant survival advantage. On the other hand, mutations within the flagellin protein that fail to form supercoiled filaments generate no thrust when such straight filaments are rotated, leading to non-motile bacteria.
Here we find another critical detail that wasn’t known before: a straight filament won’t work. It has to supercoil, so it can function like an Archimedean screw (the kind that Archimedes invented to draw water up out of a ditch). Beautiful depictions of the filament proteins arranged in geometrical works of art can be seen in Figure 1 of this open-access paper. The proteins must be packed just right so that they will not only supercoil, but quickly change between left and right orientations in a process called “polymorphic switching.” Notice how finely tuned this architecture is. If it’s not tightly balanced, it won’t work!
From the analysis of seven straight mutants in B. subtilis and two straight mutants in P. aeruginosa, we confirmed the bi-state mechanism of polymorphic switching: that only two types of subunit-subunit interactions (L-type and R- type) exist. This suggests that the wild type flagellar filaments with swimming motility must adopt an intricate “balance” in the flagellin sequence, so that the sequence does not generate a strong preference for either the L- or R- type conformation. Such a balance is required for the sharp transition of flagellar filaments switching between different waveforms during bacterial swimming and tumbling. The fact that straight phenotypes can be readily found due to single point mutations indicates that this balance is exquisitely sensitive to small changes, such as a single mutation, and can be easily tipped towards a dominant conformation, either all L or all R, which eliminates motility.
Amazing. Yet they attribute this all to evolution. They speak of how the sequence of flagellin proteins has been “tuned” by evolution. Incredibly, at the end of the paper, they appeal to convergence to claim that blind chance hit the jackpot on fine tuning three different times in all three kingdoms of life!
The bacterial flagellar filament is an exquisitely tuned system that represents evolutionary development over hundreds of millions of years. In contrast to the simplicity of the bacterial flagellum [sic], the eukaryotic flagellum, which has no homology to the bacterial one, is based upon microtubules and dynein rather than a homolog of flagellin and is currently estimated to contain more than 400 different proteins. The archaeal flagellum, which has no homology to either the bacterial or eukaryotic ones, has only recently been solved at near-atomic resolution showing how the core of these archaeal filaments is formed by a domain that is homologous to the N-terminal domain of bacterial Type IV pilin. Convergent evolution has thus yielded three very different flagellar filaments that all allow cells to swim, although by entirely different mechanisms. We are now entering a new era where the structure of such filaments can be solved at a near-atomic level of resolution using cryo-EM. We expect that the present study and future ones will yield new insights into how flagella-based swimming motility has independently arisen at least three different times in evolution using very different components, and how these convergent adaptations use very different mechanisms to achieve a similar function.
What can one say? Perhaps the best response is the title of a book by Frank Turek: “I don’t have enough faith to be an atheist.”
High-Powered Sheathed Flagella
Did you know that some bacteria have sheaths over their propeller filaments? Apparently this helps them generate higher torque and speed, and demonstrates that flagella come, like automobiles, in a variety of forms. Maybe these are the SUV’s of the bacterial world, because they can really move! Another paper in PNAS investigates bacteria in the Vibrio genus with sheathed flagella, which have not been studied as much as the unsheathed forms like in E. coli. Let’s listen to their praise of these molecular motors in their introduction:
A sophisticated chemotactic signaling system allows the bacterium to sense chemical stimuli and effectively swim toward favorable environments by a biased random walk, a combination of “runs” and “tumbles”.
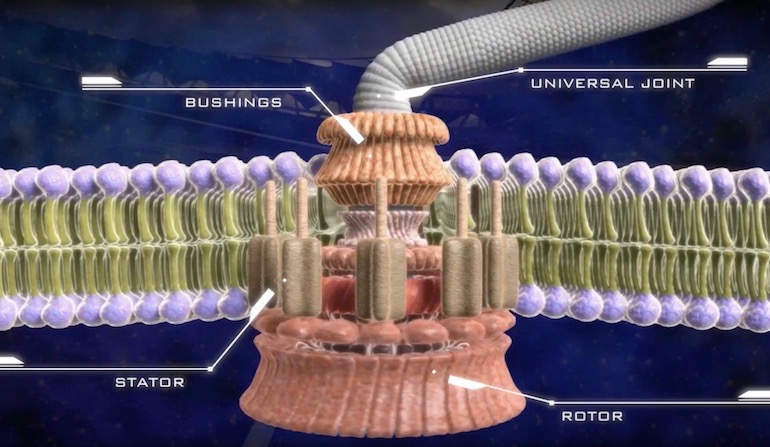
The flagellum is a large macromolecular assembly composed of a long filament, a hook, and a motor. The flagellar motor is a remarkable nanomachine embedded in the bacterial cell envelope. More than 20 different proteins are required for the assembly of the motor, which can be divided into several morphological domains. The MS ring is embedded in the cytoplasmic membrane. The C ring, known as the switch complex, and the export apparatus are located in the cytoplasmic side of the MS ring. The rod connects the MS ring and the hook and is commonly divided into the distal rod and the proximal rod. The L and P rings on the rod function as bushings at the outer membrane and at the peptidoglycan layer, respectively. The stator is the torque generator embedded in the cytoplasmic membrane…. Powered by the proton motive force, the stator generates the torque required to rotate the motor, the hook, and the filament. The stator shares several common features despite the difference in ions and proteins involved. Typically, multiple stator units work together, although a single stator is enough to generate rotational torque. Stepwise photo-bleaching of a functioning motor region revealed that the stator is highly dynamic and can associate into or dissociate from the rotor rapidly. Recent reports revealed that the stator–rotor association is dependent on conducting ions and torque load.
The authors found new parts in the sheathed flagella motor, which might not be surprising given the higher performance demands. They found a T-ring, with 13-fold symmetry, which seems to increase torque; an H-ring, which is apparently essential during motor assembly; and an O-ring, which seems to help build the sheath. These new parts are all intricately tied into the motor, just as you would expect hub lockers, the transfer case and redesigned differentials to be built to coordinate with all the other parts in a 4-wheel-drive vehicle. The diagrams of these new parts are really stunning. They look like they were made in a machine shop.
The researchers found that the sheath flexes over the filament with a sliding action. Maybe that provides flexibility like good shocks on a Jeep. The new parts of this motor add more levels of irreducible complexity to the flagellum, resulting in a high performance engine!
In comparison with the well-studied unsheathed flagellar motor in E. coli (Fig. 1 F and I), these Vibrio-specific components not only reflect the complexity of Vibrio sheathed flagellum (Fig. 5C) but also provide a solid foundation for establishing the characteristic high velocity and robustness of Vibrio motility….
These overall properties of the distal rod are consistent with its main function as a drive shaft and may allow the outer membrane-bound complex to move flexibly with the membranous sheath during flagellar rotation. Alternatively, the distance variations may reflect structures that are fixed for each motor, but differ in length among different motors.
This paper has nothing to say about evolution. We already know that the peer-reviewed journals have Behe and intelligent design on their minds — they just won’t address us by name. Fine. But really, how much more machine-shop lingo can Darwin stand?
*Many of the stunning new images of flagella have been made possible by cryo-electron microscopy, for which three inventors received the Nobel Prize in Chemistry this month. Their contributions converged in 2013 with the best images ever of the three-dimensional structure of biomolecules. “Biochemistry is now facing an explosive development and is all set for an exciting future,” the Nobel Committee said. We can look forward to sharper images of molecular machines that showcase the argument for design.
