 Human Origins
Human Origins
Apeman Waves Goodbye to Darwinian Gradualism
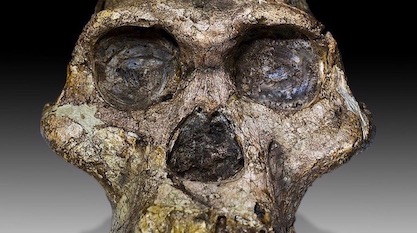
A few days ago a sensational new paleontological discovery made headlines around the globe. After 15 years of searching, and the recovery of 12,600 fossils including 230 hominin remains (Leakey Foundation 2019), finally a rather complete skull has been found and described for Australopithecus anamensis, which is the oldest and most primitive representative of the australopithecines, living 4.2-3.9 million years ago. It was generally considered to be the direct ancestor of Lucy’s species, Australopithecus afarensis, that lived in the same region 3.8-2.9 million years ago. The former species was previously known only by some fragments. Now we can finally give it a face. Actually, this face turns out to be very much ape-like, with a small chimp-sized braincase and a protruding jaw, but that is not the really interesting thing about this discovery. I will come back to that in a moment.
The Background to the Story
The fossil skull was discovered in 2016 by a native goat herder in Ethiopia’s Afar region in sediments beneath a pile of goat dung. It was excavated and described by the famous paleoanthropologist Haile-Selassie, from the Cleveland Museum of Natural History, and his colleagues (Haile-Selassie et al. 2019). The vivid story of the discovery is available in more detail at the National Geographic website (Greshko 2019). Because of the lucky circumstance that volcanic ash layers were deposited directly below and above the layers from which the skull came, this fossil could be very precisely dated to an age of 3.8 million years by radiometric methods (Saylor et al. 2019). Thus it is 100,000 years younger than the previous oldest remains from this species. Nevertheless, this skull, nicknamed MRD after its collection number, is the oldest australopithecine skull ever found. It also ranks among the very few relatively complete ones. It represents a truly remarkable discovery of tremendous scientific importance, which is already “set to become another celebrated icon of human evolution” (Spoor 2019).
“MRD has a mix of primitive and derived facial and cranial features that I didn’t expect to see on a single individual,” according to its discoverer, Haile-Selassie (Max Planck Society 2019). This means we have here another fossil that doesn’t satisfy Darwinian expectations and does not fit with any phylogenetic tree without major incongruences in the character distribution. But even this is not the really important thing about this discovery. So, what is the real surprise? The great surprise is that A. afarensis can no longer be derived by gradual anagenetic species transition from A. anamensis, as most specialists still believed until a few days ago. How come?
Because the new skull showed for the first time what the frontal bone of A. anamensis looked like, and how it differed from that of A. afarensis, scientists could finally determine the specific affinities of an isolated frontal bone (known as the “Belohdelie frontal”) that was discovered in 1981, also in the Afar region of Ethiopia (Asfaw 1987). It turned out to belong to A. afarensis, even though it is reliably dated to 3.9 million years, thus 100,000 years older than the new skull of A. anamensis. This implies that both species did overlap for a considerable period. Consequently, A. anamensis cannot have just transformed and dissolved into A. afarensis.
Such anagenetic evolution by gradual species-to-species transitions (without branching events) is actually predicted by Darwin’s theory. Therefore, we should expect to find some fossil evidence for this crucial process. But such evidence turned out to be elusive (see below), and the case of the supposed transition from A. anamensis to A. afarensis was “one of the strongest cases for anagenesis in the fossil record” (Melillo quoted in Marshall 2019, Kimble et al. 2006, Haile-Selassie 2010). This strongest case has now evaporated, and it was not only the strongest case but also the last case, as I will explain in a moment.
Unhappy, and Skeptical
Some scientists of course are unhappy with this conclusion and remain skeptical, referring to the small sample size of just two specimens (Price 2019). However, small samples are the norm in paleoanthropology, and there is also more evidence that supports this particular conclusion and contradicts even the alternative that A. afarensis branched from local population of A. anamensis by cladogenetic speciation, while other populations of the stem species “survived” this speciation event. Actually, some characters of the A. anamensis skull, especially the very pronounced cheek bones, do not fit well with an ancestral relationship to A. afarensis at all, and rather suggest that A. anamensis could be ancestral to A. africanus and the robust australopithecines (genus Paranthropus). Even though Haile-Selassie et al. (2019) still support the traditional placement of A. anamensis, they also mention that “the fact that MRD shares some neurocranial and facial morphological features with younger taxa such as A. africanus and Paranthropus — albeit considered here to be more likely to have been caused by parallel evolution — is worth further investigation in the future, as it may have considerable bearing on the origin of A. africanus and its relationship with A. afarensis.” Actually, recent phylogenetic studies of fossil hominin relationships have yielded quite different trees, of which some have A. africanus as a sister group of Paranthropus (e.g., Dembo et al. 2016). The temporal gap between A. africanus and A. anamensis would be perfectly bridged by the 3.67-million-year-old “Little Foot” skeleton of Australopithecus prometeus as a suitable link. Thus, there is considerable evidence to exclude A. anamensis not only from the stem line of A. afarensis but also from that of our own genus Homo. Together with the evidence for a temporal overlap with A. afarensis, we can safely say goodbye to this strongest fossil showcase of anagenesis.
No Exaggeration Here
But of course, the title of this article would be an exaggeration if there were not more to say about the lack of fossil support for gradualist speciation in general and anagenesis in particular. As I mentioned above, such fossil evidence has proven to be very elusive. So much so that you will only find three prominent examples in the textbooks (A. anamensis, Steinheim freshwater snails, and Globorotalia foraminifers). One of these three examples we just found to be obsolete, and the other two you will find refuted below. But first let us clarify why gradualism and its lack of empirical support is such an important issue.
Darwin’s theory of evolution directly and necessarily predicts a gradual pattern of fine-graded species-to-species transitions to climb “mount improbable,” to use Richard Dawkins’ famous metaphor. This is by no means an obsolete view from Darwin’s era, since Dawkins (2009) made very clear that “Evolution not only is a gradual process as a matter of fact; it has to be gradual if it is to do any explanatory work.” Thus, gradualism is not just some optional minor component of this theory. If gradualism is false, then Darwinism is false as well. The fossil record offers a unique window into the past that allows us to verify whether the expected gradual pattern can really be found in nature. Darwin (1859) himself was quite aware that the fossil record does not support his prediction:
Why if species have descended from other species by fine gradations, do we not everywhere see innumerable transitional forms?…Why do we not find them imbedded in countless numbers in the crust of the earth?… The number of intermediate varieties, which have formerly existed, [must] be truly enormous, … Why then is not every geological formation and every stratum full of such intermediate links? Geology assuredly does not reveal any such finely graduated organic chain; and this, perhaps, is the most obvious and gravest objection which can be urged against my theory.
This is certainly true for the major novelties in the history of life (macroevolution), which generally do not show a gradual development of new body plans, but appear quickly during abrupt “explosive” events (Bechly & Meyer 2017). But, it is also true on the minor scale of species-to-species transitions (microevolution). Darwin could still reasonably refer to the fragmentary and poorly known fossil record. He hoped that over time new paleontological discoveries might resolve this problem for his theory. However, even 160 years later this has not happened, despite the greatly expanded knowledge we have today and a “completeness of the fossil record that is rather high for many animal groups” (Foote & Sepkoski 1999, Benton et al. 2000). Therefore, Darwin’s appeal to an undersampling of the fossil record to explain away the absence of evidence for phyletic gradualism is no longer tenable. (See also my video, “How Complete Is the Current Fossil Record?”)
“Stasis Is Data”
Of course, paleontologists were quite aware of this problem that abrupt appearance of new species is the norm; or to put it another way, in the fossil record, “stasis is data” (Gould 1991). This conundrum led two American paleontologists Niles Eldredge and Stephen Jay Gould to propose their famous model of “punctuated equilibria” (Eldredge & Gould 1972). This model is often misunderstood as advocating saltational evolution, which it explicitly does not. It is just a special version of gradualism that confines the incremental evolution to an isolated small subpopulation and compresses it into a shorter period of time. It suggests that instead of continuous gradual evolution (phyletic gradualism), in reality short phases of very rapid evolutionary change were generally followed by long periods of morphological stasis: “New species arise very rapidly in small, peripherally isolated local populations by allopatric speciation, which automatically results in apparent gaps in the fossil record, because new species do not evolve in the area of their ancestors” (Eldredge & Gould 1972). This is postulating a process that is almost impossible to observe in the fossil record, forging a completely ad hoc hypothesis to explain away inconvenient conflicting evidence. The introduction to Eldredge and Gould’s famous article concludes with a revealing admission: “This idea, that theory dictates what one sees, cannot be stated too strongly.” Eldredge and Gould suggested punctuated equilibria as a general phenomenon, but it was never accepted as such within mainstream evolutionary biology. Many Darwinists rejected it and others considered it as nothing but a “minor wrinkle on the surface of neo-Darwinian theory” (Dawkins 1986). Anyway, even if “punk-eek” might explain some cases of apparent abrupt appearance of new species, it certainly cannot explain the general absence of any evidence for gradual phyletic evolution in the whole fossil record.
Unsurprisingly, Eldredge and Gould’s implicit admission of empirical failure did not meet with enthusiastic acceptance among evolutionary biologists, who instead desperately tried to find at least some evidence for Darwinian gradualism in the fossil record, though hardly with success. Gingerich (1983) acknowledged that the little fossil evidence that has been used to support the Darwinian hypothesis of phyletic gradualism “could be an artefact of time averaging” and “Rate distributions do not yet provide a definitive test of the two models.” So what little evidence has been found at all?
Update the Textbooks
Most of the few documented cases for phyletic gradualism came from fossil marine microplankton in deep-sea sediments (Ozawa 1975, Dzik & Trammer 1980, Malmgren & Kennett 1981, Benton & Pearson 2001, Pearson & Ezard 2014). Many of these cases only show minor variation within species rather than between species. Newer data furthermore suggest that cryptic diversity has to be taken into account to make sense of observed patterns of unidirectionality and rates of lineage evolution (Alizon et al. 2008). By far the most prominent example came from planktonic marine foraminifers, small protists with very diverse and beautiful shells. The case in question was the proposed gradual transition within 500,000 years between the extinct species Globorotalia plesiotumida into Globorotalia tumida, both found in Miocene marine sediments. This story you still find in many textbooks as fossil evidence for anagenesis. However, the textbooks will have to be updated because of a seminal study by Hull & Norris (2009), who carefully reanalyzed the data. They came to a very different conclusion, wonderfully captured already in the title of their article, “Evidence for abrupt speciation in a classic case of gradual evolution.” In their abstract the authors wrote, “Our findings provide an unexpected twist on one of the best-documented cases of within-lineage phyletic gradualism,” Too bad, especially since similar earlier findings had already refuted several other (weaker) cases of apparent gradual evolution in marine plankton.
Another famous textbook example for alleged gradual evolution was the fossil freshwater snails of the planorbid genus Gyraulus from the Miocene Steinheim basin in southern Germany. In the second half of the 19th century the German paleontologist Franz Hilgendorf studied the snails from this famous fossil locality. He found very different shell types that he later attributed to different species (Hilgendorf 1879). He reconstructed their presumed relationships as a transitional lineage with few side branches. He published his results with one of the earliest phylogenetic trees (Hilgendorf 1866, 1867), and thus (supposedly) the first fossil evidence for Darwin’s theory on the origin of species (Rasser 2006, Tassy 2011). Hilgendorf’s proposed transformation series was disputed by most contemporary paleontologists (Rasser 2014), but supported by later studies of Darwinian scientists (Rasser 2013). However, there exists not a single cladistic analysis of these snails, so that any rigorous support for Hilgendorf’s tree reconstruction is wanting.
Nevertheless, Hilgendorf’s findings were still celebrated as Darwinian success story in a series of papers by my former colleague Michael Rasser (2006, 2013, 2014) from the Natural History Museum in Stuttgart, Germany. He also mentioned that there were early doubts raised by some scientists (e.g., Gottschick & Wenz 1919-1922 and Wenz 1922), who questioned whether the different shell types really represented different species instead of just different morphs (so-called ecophenotypes) of the same species in the same habitat. That possibility was initially considered by Hilgendorf himself. But Rasser and other evolutionist paleontologists dismissed this critique as unfounded. They were all proven wrong by a new study of living members of the genus Gyraulus in Lake Bangong on the Tibetan Plateau (Clewing et al. 2015) as a recent analogue for the Steinheim paleohabitat. What Catharina Clewing and her colleagues discovered on the “Roof of the World” are different shell types known from the Steinheim fossils as ecophenotypic plasticity of the same living species in the same modern lake, probably induced by ecological stress. Consequently, the Steinheim snails can no longer be considered as valid evidence for anagenesis, and likely do not even represent a genuine phylogeny at all. Honi soit qui mal y pense — you will hear no Darwinist ever speak about such results of modern science.
“May He Be Shamed Who Thinks Badly of It”
Among vertebrates one of the very few examples for alleged gradual transition between two species and genera is the dentition of tarsier-like primates (Omomyidae) from the Eocene of Wyoming (Rose & Bown 1984). However, the latter authors mention that this transition happens in a mosaic fashion with greater variation preceding a shift to another character state, which at least raises justified doubts concerning the gradual pattern of this transition. Previous studies by Gingerich and colleagues on fossil mammal teeth have been largely discounted as evidence for gradualism (Gould & Eldredge 1977, Stanley 1979). More recently, Carr et al. (2017) claimed to have found evidence for anagenetic evolution in two tyrannosaurid species of the genus Daspletosaurus from the Late Cretaceous in Montana. However, the evidence does not support phyletic gradualism, but only the immediate temporal succession of two distinct but most closely related species at the same place. This can be interpreted as compatible with an ancestor-descendant lineage, but they did not find any intermediate specimens between these two distinct species. Thus, again no evidence for gradualism or anagenesis.
After the Darwin Year in 2009, celebrating his 200th birthday and the 150th anniversary of the publication of The Origin of Species, Hunt (2010) reviewed all the fossil evidence for species transitions assembled by paleontologists in 150 years of research since the time of Charles Darwin. He emphasizes that in most cases the actually observed changes in the fossil record are not directional, with very slow net rates. Based on evidence from stickleback fish in a high-resolution sequence of strata (Hunt et al. 2008), which suggest a directional change within 1,000 generations, he proposes that such change would be too rapid to be resolved in most geological sequences. How convenient, and misleading because it is not an example of species-to-species transition, but just change within a single stickleback species (Gasterosteus doryssus). Nevertheless, Hunt’s conclusion, regarding all the available fossil evidence, was startling indeed: “
The meandering and fluctuating trajectories captured in the fossil record are not inconsistent with the centrality of natural selection as an evolutionary mechanism, but they probably would not have been predicted without the benefit of an empirical fossil record.
That is a formidable example of obfuscating language. It can be translated as: the empirical data from the fossil record totally contradict the gradualist predictions of Darwin’s theory. There just is hardly any fossil evidence for directional and gradual species-to-species transitions, and especially not for anagenesis. The demise of the three textbook examples described above leaves Darwinian paleontologists empty-handed.
Left Empty-Handed
This may come as a surprise even for many critics of Darwinian evolution, because neither intelligent design proponents nor old earth or young earth creationists generally deny that neo-Darwinism may sufficiently explain low-level speciation, such as the diversification of a founding finch species into the various species of Darwin finches on the Galápagos Islands. That even such a minor phenomenon of gradualist evolution is not supported by fossil evidence gives reason for pause. Maybe we should not grant too much, too early to Darwin’s theory. Neo-Darwinian mechanisms certainly can well explain intraspecific changes of gene frequencies, like the rise of antibiotic resistance in germs, but it is unclear if the explanatory value of this process can be stretched much further. This does not imply that “God did it” as some critics of intelligent design theory often mockingly claim. But it does imply that the fossil support for neo-Darwinism is still very much exaggerated in our education system. And it suggests the need for a paradigm change in evolutionary biology, as is definitely becoming more and more evident. It is not intelligent design theorists who are the science deniers, but rather all those stubborn Darwinists. The latter still close their eyes to the ever-increasing number of anomalies that their pet theory fails to explain.
But there is a silver lining: At the conference “New Trends in Evolutionary Biology,” hosted by the prestigious Royal Society in London in November 2016, the renowned evolutionary theorist Professor Gerd Müller explicitly mentioned “non-gradual forms of transition” among his list of five explanatory deficits of the Modern Evolutionary Synthesis (aka neo-Darwinism). The other points include phenotypic novelty and phenotypic complexity. You heard that right: Everything that is really interesting in the history of life and that should be explained by Darwin’s theory, this very theory actually fails to explain, by the admission of modern evolutionary biologists themselves. No wonder that high-ranking intellectuals like Yale professor David Gelernter are giving up on a beautiful but refuted theory (Gelernter 2019).
The “beauty” of theories does not correlate with their truth. Or as theoretical physicist Sabine Hossenfelder recently asked in a new book, “Why should the laws of nature care about what I find beautiful?” (Ananthaswamy 2018). The basically dead theory of supersymmetry is a perfect example. It failed all empirical tests at LHC and will take the complete field of string theory, which depends on supersymmetry, with it into the grave. Thousands of scientists and publications wasted on a beautiful but false theory. Darwinism is about to follow their lead, and apeman Australopithecus anamensis will be remembered as one of its many undertakers.
References
- Alizon S, Kucera M, Jansen VAA 2008. Competition between cryptic species explains variations in rates of lineage evolution. PNAS 105(34), 12382–12386; DOI: 10.1073/pnas.0805039105.
- Ananthaswamy A 2018. How the belief in beauty has triggered a crisis in physics. Nature 558, 186–187; DOI: 10.1038/d41586-018-05374-9.
- Asfaw B 1987. The Belohdelie frontal: new evidence of early hominid cranial morphology from the Afar of Ethiopia. Journal of Human Evolution 16, 611–624.
- Bechly G & Meyer SC 2017. Chapter 10. The Fossil Record and Universal Common Ancestry. pp. 331-361 in Moreland JP et al. (eds). Theistic Evolution: A Scientific, Philosophical, and Theological Critique. Crossway Publ., 1008 pp.
- Benton MJ, Pearson PN 2001. Speciation in the fossil record. Trends in Ecology & Evolution 16(7), 405–411.
- Benton MJ, Wills MA, Hitchin R 2000. Quality of the fossil record through time. Nature 403, 534–536, DOI: 10.1038/35000558.
- Carr TD, Varricchio DJ, Sedlmayr JC, Roberts EM, Moore JR 2017. A new tyrannosaur with evidence for anagenesis and crocodile-like facial sensory system. Scientific Reports 7, 44942, 11 pp.; DOI: 10.1038/srep44942.
- Darwin C 1859. On the Origin of Species … . John Murray, 502 pp.
- Dawkins R 1986. The Blind Watchmaker. Norton & Company, xiii+332 pp.
- Dawkins R 2009. The Greatest Show on Earth: The Evidence for Evolution. Free Press, 480 pp.
- Clewing C et al. 2015. Ecophenotypic plasticity leads to extraordinary gastropod shells found on the “Roof of the World”. Ecology and Evolution 5(14), 2966–2979; DOI: 10.1002/ece3.1586.
- Dembo et al. 2016. The evolutionary relationships and age of Homo naledi: An assessment using dated Bayesian phylogenetic methods. Journal of Human Evolution 97, 17–26; DOI: 10.1016/j.jhevol.2016.04.008.
- Dzik J, Trammer J 1980. Gradual evolution of conodontophorids in the Polish Triassic. Acta Palaeontologica Polonica 25(1), 55–89.
- Eldredge N & Gould SJ 1972. Punctuated equilibria: an alternative to phyletic gradualism. pp. 82–115 in: Schopf TJM (ed.) Models in Paleobiology. Freeman, Cooper & Co., 256 pp.
- Foote M & Sepkoski JJ Jr 1999. Absolute measures of the completeness of the fossil record. Nature 398, 415–417, DOI: 10.1038/18872.
- Gelernter D 2019. Giving Up Darwin. CRB May 1, 2019.
- Gingerich PD 1983. Origin and evolution of species: evidence from the fossil record. pp. 125–130 in: Chaline J (ed.) Modalités, Rythmes, Mécanismes de L’Évolution Biologique: Gradualisme Phylétique ou Équilibres Ponctués? Colloques Internationaux du Centre National de la Recherche Scientifique 330.
- Gottschick F & Wenz W. 1919–1922. Die Land- und Süßwassermollusken des Tertiärbeckens von Steinheim am Albuch. Nachrichtsblatt der deutschen malakozoologischen Gesellschaft 51, 1–23; 52, 120–127; 53, 33–47; 54, 06–109.
- Gould SJ 1991. Opus 200. Natural History 100(8), 12–18; PDF.
- Gould SJ & Eldredge N 1977. Punctuated Equilibria: The Tempo and Mode of Evolution Reconsidered. Paleobiology 3, 115–151; DOI: 10.1017/S0094837300005224.
- Greshko M 2019. ‘Unprecedented’ skull reveals face of human ancestor. National Geographic Aug. 28, 2019.
- Haile-Selassie Y 2010. Phylogeny of early Australopithecus: new fossil evidence from the Woranso-Mille (central Afar, Ethiopia). Phil. Trans. R. Soc. B 365, 3323–3331; DOI: 10.1098/rstb.2010.0064.
- Haile-Selassie Y et al. 2019. A 3.8-million-year-old hominin cranium from Woranso-Mille, Ethiopia. Nature Aug. 28, 2019; DOI: 10.1038/s41586-019-1513-8.
- Hilgendorf F 1866. Planorbis multiformis im Steinheimer Süßwasserkalk. Ein Beispiel von Gestaltveränderung im Laufe der Zeit. Weber, Berlin, 36 pp.
- Hilgendorf, F 1867. Über Planorbis multiformis im Steinheimer Süßwasserkalk. Monatsberichte der Königlich Preussischen Akademie der Wissenschaften zu Berlin 1866, 474–504.
- Hilgendorf F 1879. Zur Streitfrage des Planorbis multiformis. Kosmos 5(10–22), 90–99.
- Hull PM & Norris RD 2009. Evidence for abrupt speciation in a classic case of gradual evolution. PNAS 106(50), 21224–21229; DOI: 10.1073/pnas.0902887106.
- Hunt G, Bell MA, Travis MP 2008. Evolution toward a new adaptive optimum: Phenotypic evolution in a fossil stickleback lineage. Evolution 62(3), 700–710; DOI: 10.1111/j.1558-5646.2007.00310.x.
- Hunt G 2010. Evolution in Fossil Lineages: Paleontology and The Origin of Species. The American Naturalist 176(S1), S61–S76; DOI: 10.1086/657057.
- Kimble WH et al. 2006. Was Australopithecus anamensis ancestral to A. afarensis? A case of anagenesis in the hominin fossil record. Journal of Human Evolution 51, 134–152; DOI: 10.1016/j.jhevol.2006.02.003.
- Malmgren BA, Kennett JP 1981. Phyletic Gradualism in a Late Cenozoic Planktonic Foraminiferal Lineage; DSDP Site 284, Southwest Pacific. Paleobiology 7(2), 230–240; DOI: 10.1017/S0094837300004000.
- Marshall M 2019. We’ve finally found a skull from one of our most important ancestors. New Scientist Aug. 28, 2019.
- Max Planck Society 2019. A 3.8-million-year-old fossil from Ethiopia reveals the face of Lucy’s ancestor. Phys.org Aug. 28, 2019.
- Pearson PN, Ezard TH 2014. Evolution and speciation in the Eocene planktonic foraminifer Turborotalia. Paleobiology 40(1), 130–143; DOI: 10.1666/13004.
- Ozawa T 1975. Evolution of Lepidolina multiseptata (Permian foraminifer) in East Asia. Mem. Fac. Sci. Kyushu Univ. Ser. D Geol. 23, 117–164.
- Price M 2019. Stunning ancient skull shakes up human family tree. Science Aug. 28, 2019; DOI 10.1126/science.aaz3033.
- Rasser M 2006. 140 Jahre Steinheimer Schnecken-Stammbaum: der älteste fossile Stammbaum aus heutiger Sicht. Geologica et Palaeontologica 40, 195–199.
- Rasser M 2013. Darwin’s dilemma: The Steinheim snails’ point of view. Zoosystematics and Evolution 89(1), 13–20; DOI: 10.1002/zoos.201300002.
- Rasser M 2014. Evolution in isolation: the Gyraulus species flock from Miocene Lake Steinheim revisited. Hydrobiologia 739(1), 7–24; DOI 10.1007/s10750-013-1677-4.
- Rose KD, Bown TM 1984. Gradual phyletic evolution at the generic level in early Eocene omomyid primates. Nature 309, 250–252; DOI: 10.1038/309250a0.
- Saylor et al. 2019. Age and context of mid-Pliocene hominin cranium from Woranso-Mille, Ethiopia. Nature Aug. 28, 2019; DOI: 10.1038/s41586-019-1514-7.
- Spoor F 2019. Elusive cranium of early hominin found. Nature Aug. 28, 2019; DOI: 10.1038/d41586-019-02520-9.
- Stanley SM 1979. Macroevolution: Pattern and Process. Freeman & Co., 332 pp.
- Tassy P 2011. Trees before and after Darwin. J. Zool. Syst. Evol. Res. 49(2), 89–101, DOI: 10.1111/j.1439-0469.2010.00585.x.
- The Leakey Foundation 2019. A 3.8-Million-Year-Old Fossil From Ethiopia Reveals the Face of Lucy’s Ancestor. Leakey Foundation Aug. 28, 2019.
- Wenz W 1922. Die Entwicklungsgeschichte der Steinheimer Planorben und ihre Bedeutung für die Deszendenzlehre. Natur und Museum 52, 135–158.
Photo: Australopithecus africanus skull, by José Braga; Didier Descouens [CC BY-SA 4.0], via Wikimedia Commons.

