 Evolution
Evolution
From Swamidass on Chloroquine Resistance, a Response that Doesn’t Respond


On a given subject that is being debated, just because someone writes something in response it doesn’t necessarily mean he has really responded to the relevant arguments. On his message board at Peaceful Science, biologist Joshua Swamidass has authored a reply to our comments on chloroquine resistance. The context is a discussion of his review in Science, co-authored with Nathan Lents and Richard Lenski, of Michael Behe’s new book, Darwin Devolves. What’s most interesting about the reply is not what it does say but what it doesn’t say.
Reviewing the Issue at Hand
As already discussed at Evolution News, the main point on chloroquine resistance was that Behe’s new book does not talk about that subject, apart from two short passing sentences — fewer words than Swamidass et al. use in seeking to rebut him on the same topic in their review! Regarding the mutations needed for chloroquine resistance, their review attributes positions to Behe that, while he may agree with them, he simply does not address or argue for in Darwin Devolves. Their review puts words into his book he didn’t write.
The second important point was that Swamidass and his co-authors twice claimed that Behe’s book fails to mention particular sources that they believe challenge Behe’s arguments on chloroquine resistance. The two sources were Summers et al. (2014) and responses to him by Durrett and Schmidt in the journal Genetics. But as we noted, Behe has interacted extensively with those specific sources and believes that they ultimately lend strong support to his position.
Swamidass et al. might disagree with Behe on chloroquine resistance, but they can’t reasonably claim he has ignored these sources. As a consequence, the Science review was very unfair to claim that Behe “ignores evidence” and “avoids evidence that challenges him.” As John West has discussed, declining to participate in Swamidass’s Peaceful Science forum is hardly evidence that one is refusing to engage with critics.
Which leads to the final point, which was to inquire why Behe did not mention these sources in his latest book. The answer is very simple: Behe does not mention these sources in Darwin Devolves because chloroquine resistance is not a topic of his latest book. There was no reason for him to mention these sources.
What Swamidass Doesn’t Say
In response, Swamidass apparently has virtually nothing to say about these three main points in critique of his review. Instead, he has asserted that simply by writing substantive responses to the Science review, Evolution News is acting like a “PR machine,” and he proceeds to rehash old substantive questions about Behe’s arguments on chloroquine resistance from his previous book The Edge of Evolution. That book came out 12 years ago. Those debates happened years ago. They are not relevant to his present book.
To review:
- Swamidass et al. made distasteful accusations against Behe in Science, alleging that Behe “ignores evidence” and “avoids evidence that challenges him,” and cited two papers regarding chloroquine resistance in support of their accusation.
- We responded by pointing out that Behe isn’t talking about chloroquine resistance in his book and therefore it’s strange and unwarranted to demand that he should have mentioned these sources. Nonetheless, Behe has engaged with these sources extensively in the past and has hardly ignored them. In fact, he thinks these sources ultimately support his arguments!
- Swamidass in his reply responds to none of our points.
Well, perhaps Swamidass vaguely responds to our first main point — but only by demanding that Behe should have covered chloroquine resistance in his present book because of the fact that some people disagreed with what he wrote about it in his previous book. So it’s almost as if Dr. Swamidass’s position has shifted from his review where he claimed (incorrectly) that in the new book Behe “doubles down” on arguments about chloroquine resistance, to his reply to us, where he now seems to admit Behe really didn’t talk about this topic in Darwin Devolves, but says he should have. Swamidass quotes Laurence Moran:
“The recent paper by Summers et al. (2014) shows that seven of the chloroquine resistant strains that have been observed have at least four mutations and some of them are relatively neutral. This refutes and discredits the scenario that Michael Behe put forth in his book. Sandwalk: Understanding Michael Behe 2”
Yes, I [Swamidass] know that Behe responded (unconvincingly) to Moran. In his book, he should have at minimum acknowledged that several scientists have engaged with his argument and found it lacking for several reasons. Yes Behe disagrees with them, but in his book Behe has to acknowledge that his claims are totally disputed; he can’t just assert that he has made his case. His Devolution case depends on him being right here, so it is a consequential error to have left out an explanation of this problem with his case.
It’s not as if in Darwin Devolves Behe fails to acknowledge that people dispute his claims. On the contrary, he spends big chunks of the book addressing ID critics and their arguments: the index lists 17 pages where “critics” of intelligent design are discussed, and that doesn’t include 18 pages in an appendix where Behe responds to criticisms of Darwin’s Black Box, as well as the names of well-known ID-critics such as Richard Dawkins, Jerry Coyne, Daniel Dennett, Russell Doolittle, Douglas Futuyma, Stephen Jay Gould, Eugene Koonin, Richard Lenski, Alan Leshner, Michael Lynch, Nicholas Matzke, Kenneth Miller, the National Center for Science Education, Mark Pallen, Joseph Thornton, and Neil deGrasse Tyson — who together cover 80 pages in the index. But it’s still unclear why Behe should have mentioned Summers et al. (2014) in his new book, since his case for “devolution” does not depend on chloroquine resistance! It’s obviously unfair to hold Behe to a rule where any book he writes must recognize every past dispute about his arguments, whether that dispute was in one of Behe’s books or a blog post, regardless of whether it’s a topic covered in the new book.
What Swamidass Does Say
If Swamidass doesn’t respond to these points, then what does he say? He quotes a blog post from Arthur Hunt from back in 2010. Hunt claimed that Behe had no basis for inferring that the rarity of chloroquine resistance stemmed only from a “double-mutation” trait and that other mutations may have been involved. The passage is not really relevant to any of the main points here. A quick look at Behe’s arguments in The Edge of Evolution and the literature will show that Behe’s positions are well founded. Here is what Swamidass quotes from Hunt:
I’ll close this essay by noting one source of error on Behe’s part. As I have discussed, Behe asserts that the probability associated with a “CCC” is 1 in 10^20. Where does this number come from? From footnote 16 in the first excerpt given above — White, N. J. 2004. Antimalarial drug resistance. J. Clin. Invest. 113:1084-92. Here is the actual passage from the review by White that mentions the number 10^20:
“Chloroquine resistance in P. falciparum may be multigenic and is initially conferred by mutations in a gene encoding a transporter (PfCRT) (13). In the presence of PfCRT mutations, mutations in a second transporter (PfMDR1) modulate the level of resistance in vitro, but the role of PfMDR1 mutations in determining the therapeutic response following chloroquine treatment remains unclear (13). At least one other as-yet unidentified gene is thought to be involved. Resistance to chloroquine in P. falciparum has arisen spontaneously less than ten times in the past fifty years (14). This suggests that the per-parasite probability of developing resistance de novo is on the order of 1 in 10^20 parasite multiplications.”
Recall that Behe equated one CCC with a double mutation, presumably based on other work showing that two point mutations in the PfCRT gene are associated with durable resistance in the parasite. But White is not talking about double mutations in PfCRT when he tosses out the number 10^20. Rather, he is speculating about the frequency of occurrence of a multigenic trait that involves two or three genes, and more (perhaps many more) than two mutations. In other words, Behe’s use of this citation to argue that the natural frequency of occurrence of a double mutation in PfCRT is 10^20 is inappropriate. This is one reason (not the only reason, but one) why Behe’s claims are so out of touch with reality.
The key inaccurate phrases from Hunt are where he says Behe necessarily “equated one CCC with a double mutation” and the notion that other mutations beside the “double mutation” are important factors in determining the rarity of chloroquine resistance.
Again, these really aren’t the topics at hand and they don’t have anything to do with mitigating the mis-statements in the Science review of Darwin Devolves. But Swamidass wants to talk about them, so fine.
Fine, Let’s Talk
First, Behe’s official definition of a chloroquine complexity cluster, or CCC, doesn’t specify that it necessarily only requires a “double mutation.” As he wrote:
Species in which there are fewer living organisms than malaria (again, other things being equal) will take proportionately longer to develop a cluster of mutations of the complexity of malaria’s resistance to chloroquine. Let’s dub mutation clusters of that degree of complexity — 1 in 1020 — “chloroquine-complexity clusters,” or CCCs. (The Edge of Evolution, p. 60)
Here, in defining a CCC, Behe doesn’t specify exactly what is involved in producing it other than some “cluster of mutations.” The details of that ‘cluster’ had not been nailed down by scientific research at the time he wrote The Edge. The key point for Behe’s argument isn’t how a CCC is generated, but that whatever is required to produce a CCC, it apparently takes 1020 trials.
In The Edge of Evolution, when Behe investigated what was “required for the primary activity by which the protein confers resistance” (p. 49, emphases added), he found two mutations were needed. This didn’t come from White’s paper. It came from papers Behe cited in Cell and Nature which indicated that sequenced genomes of strains of resistant P. falciparum from around the world showed that two mutations seemed to be consistently required for chloroquine resistance — found at positions 76 and 220 in the protein PfCRT. Behe thus predicted:
Since two particular amino acid changes occur in almost all of these cases [of chloroquine resistance], they both seem to be required for the primary activity by which the protein confers resistance. The other mutations apparently “compensate” for side effects caused by these two primary mutations. (The Edge of Evolution, pp. 49, 51)
Note that Behe’s last sentence there acknowledges that “other mutations” are also involved in producing the resistant strains, but that they play compensatory roles. This is consistent with White’s statement (cited by Swamidass in quoting Hunt) that chloroquine resistance might be multigenic. So Behe didn’t disallow the possibility that other mutations and other genes are involved in a CCC.
Again, Behe makes it clear that a CCC involves a “cluster” of mutations, and is not necessarily limited to mutations only in one “particular protein”:
Although some other mutations in some other proteins are thought to contribute to chloroquine resistance,22 none are nearly as effective as that in PfCRT. That means that of all of the possible mutations in all of the, different proteins of malaria, only a minuscule number have the ability to help at all against chloroquine, and only one, PfCRT, is really effective. Natural selection gets to choose from a staggering number of variations, yet at best only a handful help. So a CCC isn’t just the odds of a particular protein getting the right mutations; it’s the probability of an effective cluster of mutations arising in an entire organism. (The Edge of Evolution, p. 62)
These passages from The Edge of Evolution show that Behe acknowledges that chloroquine resistance might involve “other mutations” in other genes beyond the two mutations in the protein PfCRT, but he says they are likely “compensatory” mutations. This means that they are secondary mutations that yielded individual selectable benefits, and aren’t the direct causal mechanism of chloroquine resistance. Behe argues that only mutations in PfCRT are “really effective” to generate resistance.
(Update: The passages quoted here also refute Swamidass’s misstatements about Behe’s views, where he says that Behe “think [sic] that CCC is just two mutations” and asserts “For his [Behe’s] argument to work, clinical resistance to malaria must require two and only two mutations.” This is not at all what Behe thinks and Swamidass’s descriptions of Behe’s arguments are contradicted by Behe’s words, quoted here, in The Edge of Evolution.)
The literature agrees with Behe. His citation 22 in the passage quoted above is to Le Bras and Durand (2003) which suggests that chloroquine resistance might also involve mutations in the genes pfmdr1 and pfcg2. However, that paper finds that these are “associated mutations” whereas the pfcrt mutations are the ones that are “causal.” Similarly, Hayton and Su (2004) suggest mutations in pfmdr1 are “compensatory” and “secondary” but the “key role” in chloroquine resistance is played by the gene pfcrt:
These observations suggest that pfcrt plays a key role in CQR [chloroquine resistance] and that pfmdr1 may play a secondary role by modulating CQ response. One possibility is that mutations in pfmdr1 are part of the compensatory changes in response to deleterious effects caused by mutations in pfcrt. Based on the mutant pfcrt haplotypes known so far, it is likely that simultaneous multipoint changes in pfcrt are necessary to confer CQR.
Thus, the other gene identified by White, pfmdr1, is not thought to be required for chloroquine resistance but likely involves secondary compensatory mutations. Chloroquine resistance might involve multiple mutations in multiple genes — a point acknowledged by Behe — but the key changes necessary to generate resistance appear to be two mutations in a single gene, pfcrt.

This leads to the second point: In The Edge of Evolution, Behe wants to understand why chloroquine resistance is so rare — i.e., why White has observed that it occurs in fewer than 1 in 1020 replications. To explain this, Behe is less interested in secondary or compensatory mutations (that probably individually confer benefits and can arise in a stepwise manner after chloroquine resistance has already evolved) than he is in understanding the primary causal mutations. He predicts that the “primary activity by which the protein PfCRT confers resistance” requires two “primary mutations” — a claim that is consistent with White’s statement that chloroquine resistance is “initially conferred by mutations in a gene encoding a transporter (PfCRT).” These “other mutations” aren’t crucial for initially creating resistance, and might arise rapidly in a stepwise manner to fine-tune and improve resistance after chloroquine resistance first evolves.
Thus, if we want to account for the peculiar rarity of chloroquine resistance, then we need to investigate the potential need for “simultaneous” mutations. In The Edge of Evolution, Behe explains that for chloroquine resistance “presumably … two [mutations] are needed” because “if a single mutation could help chloroquine resistance would originate much more frequently.” (p. 59) This is precisely what Hayton and Su (2004) found when they wrote that “simultaneous multipoint changes in pfcrt are necessary to confer CQR [chloroquine resistance].” And it’s exactly what Summers et al. (2014) found when they reported that “the minimum requirement for (low) CQ transport activity in both the ET and TD lineages of CQR [chloroquine resistant] PfCRT is two mutations.”
The point is this: Yes, White did mention that other genes might be involved in chloroquine resistance, and yes, Larry Moran suggests other mutations are involved in the pathway. Behe too acknowledges that other mutations, including mutations in proteins apart from PfCRT, might be involved in a CCC. But Behe notes that these are probably compensatory mutations, not absolutely required for chloroquine resistance, and the literature cited above confirms this. Such beneficial mutations can evolve quickly in a stepwise manner, and they aren’t what is making this trait rare.
Indeed, Summers et al. (2014) also found that the later mutations in pathways of chloroquine resistance, after some degree of resistance had first arisen, have beneficial effects, including beneficial compensatory effects. This is why Larry Moran is wrong to claim that this paper necessarily showed many neutral mutations (in addition to the initial double-mutation) were required to produce resistance.
Looking at Figure 3 of the paper, reprinted below, you can see that all of the mutational pathways that yield the 7 different chloroquine resistant phenotypes start off with a double-mutation that confers some minimum level of chloroquine resistance (these are D39 + D38 Figure 3A, or D38 + D33 in Figure 3B). This is why Summers et al. state that “A minimum of two mutations sufficed for (low) CQ [chloroquine] transport activity… the minimum requirement for (low) CQ transport activity … is two mutations.” This initial double-mutation is what validates Behe’s predictions about the reason for the rarity of the pathway:
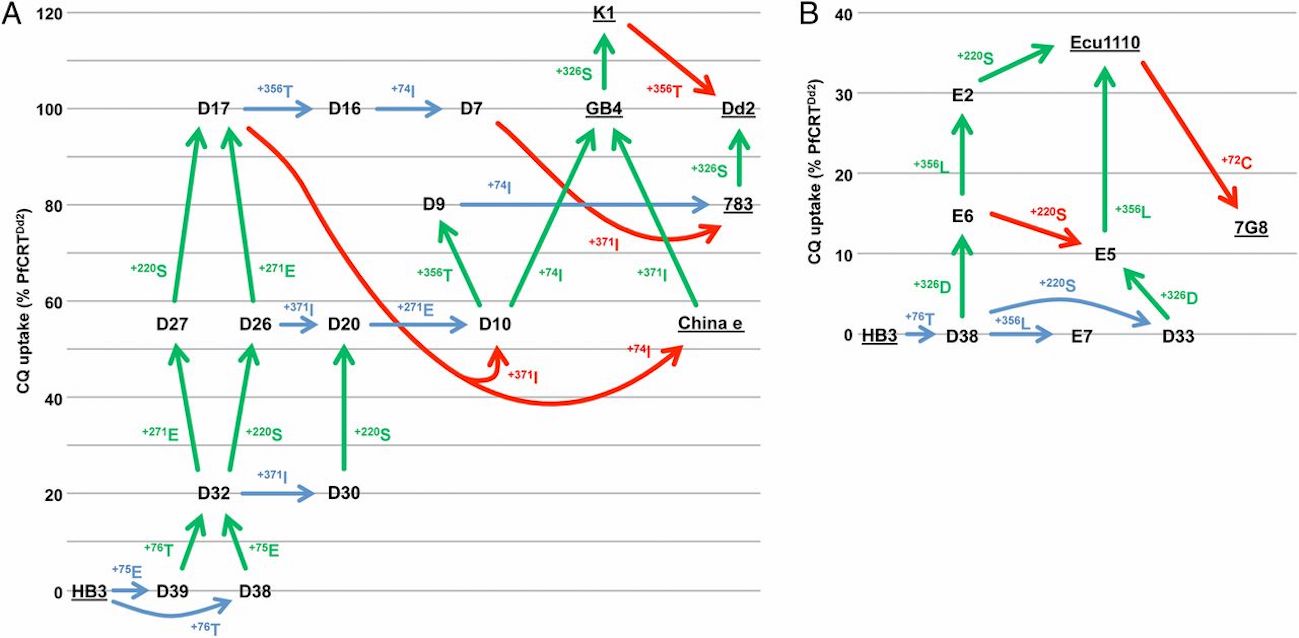
Reprinted from Summers et al., “Diverse mutational pathways converge on saturable chloroquine transport via the malaria parasite’s chloroquine resistance transporter,” Proceedings of the National Academy of Sciences, 111 (17): E1759-E1767, Copyright (2014), National Academy of Sciences. Use of figure does not imply any endorsement by PNAS or NAS.
Later mutations in the pathways can lead to higher levels of resistance by improving chloroquine uptake efficiency. These mutations, colored green in the figure, seem to be beneficial and can therefore evolve quickly in a stepwise manner. They are probably not what is making this trait rare. But some of the later mutations in the pathway above are colored either blue for neutral, or red for deleterious. Hence Larry Moran’s claim that the pathways require neutral mutations. Perhaps they do, but this paper has not established that that this is the case.
In Summers et al. (2014), all fitness effects were evaluated with respect to measuring chloroquine uptake in oocytes of Xenopus laevis, a frog. Take note: They aren’t studying effects of these mutations in vivo in the parasitic protist that causes malaria, Plasmodium. After the initial double-mutation that they report confers some minimum level of resistance, we don’t really know how these later mutations overall affect the organism. It is known that many mutations in PfCRT cause problems for Plasmodium, and thus Summers et al. (2014) note that mutations that appear to be neutral or deleterious with respect to chloroquine uptake may likely be compensatory mutations in Plasmodium that provide a selectable benefit to the organism:
Some of the intermediate haplotypes with reduced CQ transport activities may in fact represent favorable tradeoffs between conferring a moderate level of CQ resistance and maintaining the normal physiological role of the protein, which is essential but as yet unknown.
Additionally, because of epistasis, an observed mutation might not always have the same negative or neutral effect in all contexts. For example, in Figure 3A, mutation D17 –> D10 involves a major drop in chloroquine uptake activity in one context (see Fig. 3) but in another context the paper found it “did not alter the ability of the protein to transport CQ.” Unfortunately Summers et al. can’t establish that these mutations that occur later in the pathway really are neutral or deleterious to the organisms because they didn’t study these intermediates stages in Plasmodium in vivo to understand their overall effects and benefits to the organism. But they state that these mutations (like +371 or + 74I) probably had compensatory benefits:
First, each mutation either maintains or increases the capacity of PfCRT for CQ transport; hence the parasite is not reliant on existing and/or simultaneous changes in other genes (e.g., those encoding PfMDR1, PfMRP1, and PfMRP2) to avoid periods of reduced resistance to CQ. Second, it describes a pathway by which one or more compensatory changes (e.g., perhaps R371I and/or M74I) could arise at an early stage to maintain the normal physiological function of the protein while it develops the ability to transport CQ.
Behe too anticipated that other compensatory mutations were involved in producing a CCC. He notes that these compensatory mutations are not absolutely required to generate chloroquine resistance, and the literature cited above confirms this.
Most likely these pathways involve multiple mutations, virtually all of which individually conferred some selectable benefit to the organism, whether by directly increasing chloroquine transport activity or providing some compensatory benefit. But if Behe wants to account for the extreme rarity of a CCC, it’s appropriate for him to seek out the need for simultaneous mutations. That is because these beneficial mutations (whether in pfcrt or other genes) aren’t what is making chloroquine resistance so incredibly rare. This need is exactly what he finds in the first two mutations in the gene pfcrt, and the double-mutation there is probably what accounts for White’s observed rarity of chloroquine resistance occurring in 1 in 1020 cells. So while Behe did not “equate” a CCC with a double-mutation that only occurs in 1 in 1020 replications, the data suggest that it’s the double-mutation required in the protein PfCRT that primarily accounts for the extreme rarity of this trait.
Summers et al. (2014), other papers, and even Behe himself correctly suggest that other mutations either increase chloroquine resistance or provide compensatory benefits, and can thus be added in a sequential manner that doesn’t strongly increase the rarity of the trait. More than anything else, it’s probably that double-mutation that’s required to start the pathway at its very beginning that makes it so rare. Once you get that rare double-mutant at the beginning, the rest of the pathways probably follow much more easily. Given that this double-mutation’s unlikelihood is likely dominating the factors that make this trait rare, there seems nothing inappropriate with Behe’s inferring that a double-mutation like that required by a CCC probably arises in about 1 in 1020 replications.
In other words, when it comes to explaining the rarity of this trait, Behe seems to be right to focus on the initial double-mutation — not Hunt who says we must look at beneficial compensatory mutations in other genes, nor Moran who wrongly claims that it’s been affirmatively established that later mutations in the pathway are neutral (something we simply don’t know to be the case).
What Is Behe’s Greater Argument?
Yet all of the above is not really crucial to Behe’s greater argument in The Edge of Evolution, which did not turn upon whether a CCC required one mutation, two mutations, or fifty mutations in fifty different genes. He said that a CCC “presumably” required two simultaneous mutations, but it wasn’t a crucial plank in his argument.
Behe’s argument was simply to observe, on the basis of White’s public health data, that chloroquine resistance arises in 1 in 1020 cells. That’s a data point. He then asked a hypothetical question: If one CCC requires 1020 replications, what would happen if there were a trait that was as complex as a “double-CCC”? Such a trait, Behe argued, would require 1040 cells to arise, which is more cells than have lived over the course of the history of the Earth. This, he concluded, would pose a problem for Darwinism:
[A] double CCC is a reasonable first place to draw a tentative line marking the edge of evolution for all of life on earth. We would not expect such an event to happen in all of the organisms that have ever lived over the entire history of life on this planet. So if we do find features of life that would have required a double CCC or more, then we can infer that they likely did not arise by a Darwinian process. (The Edge of Evolution, p. 63)
While Behe predicted that a single CCC probably required a “double mutation,” his argument did not require this to be the case. A previous response at Evolution News already pointed this out:
Despite the protests from critics, it turned out that Behe reasonably inferred that chloroquine resistance not only requires multiple mutations, but multiple mutations must be present before resistance can evolve. His critics mistakenly thought this was a crucial plank in his argument, when it wasn’t. But Summers et al. (2014) showed that Behe’s ID-inspired suspicions were right all along. (Emphasis added.)
In any case, Behe is getting heat here when he should be getting credit. As already pointed out, he turned out to correctly predict that multiple simultaneous mutations were required for chloroquine resistance and that’s what exactly Summers et al. (2014) conclusively showed.
Swamidass’s response doesn’t address our main arguments, but he indicates he is impressed by what Darwinian mechanisms accomplished in generating chloroquine resistance. If he’s impressed with these examples, that’s fine. But keep in mind that Behe long ago recognized that this trait is within the “edge of evolution.” Just because some traits are within the edge of evolution does not mean that all are. Much of Behe’s work also identifies traits that are beyond the reach of unguided evolutionary mechanisms. To learn more about them, check out Darwin Devolves.
Photo: Xenopus laevis, by Brian Gratwicke [CC BY 2.0], via Wikimedia Commons.
